
Rebecca Dekker
PhD, RN
Evidence on: Pitocin® During the Third Stage of Labor
Written by Rebecca Dekker, PhD, RN and Anna Bertone, MPH on June 4, 2020.
Table of Contents
- How Has Care During the Third Stage of Labor Changed Over Time?
- Oxytocin and Synthetic Oxytocin (Pitocin®)
- How Do Oxytocin and Pitocin® Work in the Body?
- What Is Postpartum Hemorrhage?
- What is the Definition of Primary Postpartum Hemorrhage?
- How Do Care Providers Assess Blood Loss?
- How Common Is Primary Postpartum Hemorrhage?
- Why is the Rate of Postpartum Hemorrhage Rising in Recent Years?
- What Factors Increase the Risk of Postpartum Hemorrhage?
- What Is the Normal Length of the Third Stage of Labor?
- What Is the Evidence on Management of the Third Stage of Labor?
- Evidence on Best Practices to Use With Expectant Management
- Evidence on Best Practices to Use With Active Management
- Are There Any Trainings Health Care Professionals Can Take to Improve Outcomes With PPH?
- Recent Practice Guidelines
- Conclusion
The third stage of labor, or placental stage, begins after the baby is born and ends after the birth of the placenta (sometimes called the “afterbirth”). It follows the first two stages of labor, which you can think of as the dilation (or opening) stage and the pushing stage. In practice, there are three main approaches to care for the birth of the placenta (Begley et al. 2019):
- Expectant management is also called physiological, conservative, passive, “watchful waiting,” or “hands-off” management. This approach to caring for the third stage of labor is popular in some European countries, New Zealand, and in midwife-led births in Ireland, the United Kingdom (U.K.), and the United States (U.S.). The care provider waits while the birthing person’s uterus continues to contract after the baby is born. These contractions cause the placenta to gradually separate from the uterus and then the placenta is pushed out or birthed with the aid of gravity.
With the expectant management approach:
- Care providers do not give Pitocin® (a synthetic form of the hormone oxytocin) or another medication that causes the uterus to contract (called a uterotonic) to prevent heavy bleeding before it occurs. Instead, they closely monitor the mother after birth and treat excess bleeding with a uterotonic medication if it occurs. Sometimes providers skilled at expectant management will take actions to support the birthing person’s natural release of oxytocin, for example through immediate skin-to-skin contact, encouraging opportunities for breastfeeding/chestfeeding or another form of nipple stimulation, and ensuring a warm, calm, private birth environment.
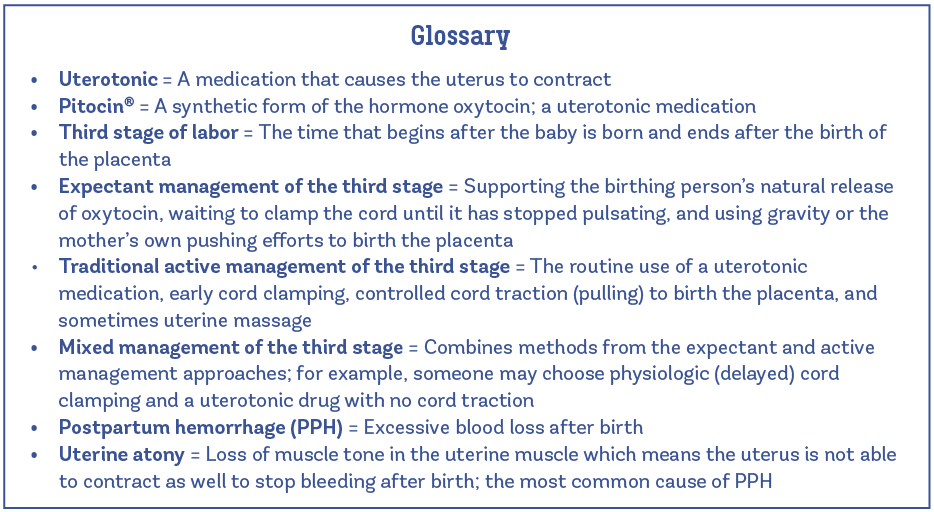
- Care providers do not clamp or cut the umbilical cord until the placenta is birthed, or at least until the cord has stopped pulsating (or has “gone white”).
- Care providers do not use traction (controlled pulling) on the umbilical cord, although some providers do use gentle traction once the placenta is visible in the vagina ready to be birthed.
- Active management is also called “hands-on” management, where the provider uses different interventions to try to prevent severe blood loss after birth, known as postpartum hemorrhage (PPH). This management approach came about in an attempt to reduce PPH, which is the leading cause of maternal deaths in countries defined as “low-income” by the World Bank, and accounts for more than a quarter of all maternal deaths globally (Vogel et al. 2019). The U.S. Centers for Disease Control (CDC) estimates that PPH accounts for about 11% of pregnancy-related deaths in the U.S. Surveys from the U.S. (Schorn et al. 2017) and the U.K. (Farrar et al. 2010) have found that physicians are much more likely than midwives to “always” or “usually” use active management.
With traditional use of active management:
- Care providers give Pitocin® in the third stage or another uterotonic drug just before, with, or after the birth of the baby to help the uterus contract.
- Care providers clamp the umbilical cord early, before the cord has stopped pulsating.
- Care providers use controlled traction on the umbilical cord with counter-pressure on the uterus to aid the birth of the placenta.
- Mixed management is sometimes called “combined” management. It is a mixture of some of the components from expectant management and some of those from active management.
- There are many variations, but one example of mixed management might include giving Pitocin® in the third stage, waiting to clamp the umbilical cord until it has stopped pulsating, and remaining “hands-off” as the birthing person expels the placenta with the aid of gravity.
- It’s important to understand that while the traditional definition of active management is a package of three components (giving a uterotonic, early cord clamping, and controlled cord traction), more modern definitions of active management focus mostly on giving the uterotonic, and could be described as mixed management. In other words, routine early cord clamping and controlled cord traction are no longer considered essential components of active management (WHO, 2019; Hofmeyr et al. 2015).
A small qualitative study found that pregnant people are often given little or no information from their care providers about their options for birthing the placenta (Reed et al. 2019). When care providers did offer information, it tended to reflect their own preferences or usual practice style rather than a true discussion of options. This is concerning, because pregnant people express diverse preferences for the third stage of labor—some consider expectant management a safer process and an essential element of natural (physiologic) birth, while others consider active management to be the safe route that prevents complications and helps you avoid further interventions.
In this Evidence Based Birth® Signature Article, we ask: Who is at lower risk for severe bleeding after birth and who is at higher risk? What is the evidence for expectant versus active or mixed management and what are the potential benefits and risks of each approach?
How Has Care During the Third Stage of Labor Changed Over Time?
Historically, the focus was on treating postpartum hemorrhage (PPH) when it occurred, not intervening in the third stage of labor an attempt to prevent PPH. Midwives treated PPH after-the-fact with vaginal packing or applying hot vaginal douches, with limited success.
The first effective treatment for PPH came into use around the 16th century (Chamberlain, 2006). Midwives discovered that extracts of ergot (a fungus) could cause the uterus to contract and stop excessive bleeding. So, if a woman appeared to be losing too much blood after birth, she would drink extracts of ergot as a “labor tea” to stop the bleeding. Since ergot worked by causing the uterus muscles to contract, it was the first uterotonic drug used to treat PPH.
Oliver Prescott, a physician in Massachusetts, published an official dissertation on the use of ergot to treat PPH in 1813. About 100 years later, in 1912, Dr. Barry Hart of Edinburgh made the case that ergot should be used before PPH, as a way to prevent excessive bleeding before it occurs (Chamberlain, 2006). In other words, he proposed active management of the third stage of labor by giving birthing people a uterotonic drug to prevent PPH. This idea caught on, especially in the 1950s, after studies found a reduction in PPH with the routine use of ergot injections in the third stage of labor. However, ergot was also linked to side effects in some people, including high blood pressure, nausea, vomiting, headache, and abdominal pain.
Today, the World Health Organization still recommends ergot preparations as an effective uterotonic drug for birthing people without high blood pressure disorders (WHO, 2018). But in settings where multiple uterotonic options are available for active management of the third stage of labor, the WHO recommends synthetic oxytocin over the other options.
Oxytocin and Synthetic Oxytocin (Pitocin®) in the Third Stage of Labor
Synthetic oxytocin first came into use in the 1950s, much later than ergot. Soon after, in the 1960s, active management (uterotonic drug plus immediate cord clamping and controlled cord traction) became popular in many hospital settings to try and prevent postpartum hemorrhage (Aflaifel and Weeks, 2012).
The hormone oxytocin was discovered in 1906 when Sir Henry Dale of London found that an extract from the human pituitary gland, a pea-sized structure at the base of the brain, caused uterine contractions in a pregnant cat (Magon and Kalra, 2011). He named the hormone “oxytocin” after the Greek words for “quick birth.” Then, in 1953, a New York biochemist named Vincent du Vigneaud published the chemical structure of oxytocin, work for which he received the 1955 Nobel Prize in Chemistry (du Vigneaud et al. 1953). Research on oxytocin in the early 1950s used extracts from cow and pig pituitary glands. Today, commercially sold oxytocin is prepared synthetically in laboratories.
Pitocin®, a synthetic form of oxytocin, is commonly used in clinical practice to start (induce) and speed up (augment) labor, as well to help the uterus contract after birth in active management. In some countries, such as Australia, they use an equivalent drug called Syntocinon®. Pitocin® and Syntocinon® are brand names of synthetic oxytocin, while the generic form of the drug is simply called “oxytocin.”
Other uterotonics are also used for PPH prevention, including carbetocin, prostaglandin analogues such as misoprostol, ergot alkaloids, or combinations of these (oxytocin plus ergometrine, or oxytocin plus misoprostol) (Vogel et al. 2019). This Evidence Based Birth® Signature Article focuses on synthetic oxytocin (Pitocin®) as the uterotonic in the third stage of labor.
How Do Oxytocin and Pitocin® Work in the Body?
To understand why uterotonics are used in management of the third stage of labor, it helps to understand more about how oxytocin and Pitocin® work in the body. Below, we refer to the body’s own naturally produced (endogenous) oxytocin as oxytocin and synthetic oxytocin as Pitocin®.
In 2019, researchers published a systematic review of 20 studies that measured oxytocin levels at different points during pregnancy and childbirth (Uvnäs-Moberg et al. 2019). During pregnancy, they found that blood levels of oxytocin gradually rise, increasing by 3 to 4 times. Levels of oxytocin rise even more in labor, when pulses of the hormone become larger and more frequent to contract the uterus and help the progress of labor. The maximal frequency of pulses was found to be 3 pulses per 10 minutes (not tied to the frequency of contractions) just before the birth. There is a large pulse of oxytocin during the actual birth when the fetal head emerges, and pulses continue during the third stage of labor with the birth of the placenta.
The presence of the body’s own oxytocin or receiving Pitocin® is critical to preventing PPH in the third stage of labor. After birth, oxytocin or Pitocin® continues to cause the birthing person’s uterus to contract in order to expel the placenta. With each contraction, the movement shears the placenta from the uterine wall (OpenStax, Anatomy & Physiology, 2016). There is an upward “wave of separation” that causes the upper part of the placenta to detach last (Funai et al. 2019). When it detaches, this leaves a placenta-sized wound on the inside of the uterus. Without effective contractions, the uterine blood vessels are left wide open and enormous amounts of blood can be lost very quickly. But when the birthing person’s oxytocin or Pitocin® continues to cause contractions, this causes the uterus to clamp down with pressure on the bleeding blood vessels where the placenta was attached and stop the bleeding at the placenta wound site—helping to prevent PPH.
How Are They Similar and How Are They Different?
Oxytocin and Pitocin® are identical in chemical structure, which is comprised of a chain of nine amino acids (called a nonapeptide). However, they don’t act on the body in the same way. In addition to causing uterine contractions that help to birth the placenta and prevent PPH, the mother’s oxytocin is also released in the brain, where it reduces anxiety, stress, and pain. This is a major difference between oxytocin and Pitocin®: the birthing person’s own oxytocin levels rise in both blood and brain fluid, but Pitocin® does not cross into the brain because of the blood brain barrier (Meisenberg an Simmons, 1983). Interestingly, researchers are now studying intranasal (nose-to-brain) delivery of synthetic oxytocin as a way of crossing the blood brain barrier and treating different brain diseases (Quintana et al. 2018).
Another difference is that when Pitocin® is given in high doses during labor, it can cause more frequent, longer, and more painful contractions. Researchers have found that giving Pitocin® infusion in doses up to 9 milliunits (mU) per minute leads to similar levels in the blood as seen with physiologic labor, whereas doses between 10-16 mU per minute raise levels to double those of physiological labor (Uvnäs-Moberg et al. 2019). The optimal dosing regimen for giving Pitocin® in practice is controversial; UpToDate® guidance for clinicians states that most protocols limit oxytocin infusion during labor with a live fetus in the third trimester to no more than 40 mU/minute (Grobman et al. 2019). In the future, we may see more research on giving Pitocin® infusion in pulses during labor, which would be more like the body’s own way of releasing oxytocin.
Oxytocin in the body (both natural and synthetic) causes the uterus to contract by binding with oxytocin receptors on the cell surface. When there is a lot of oxytocin, the body compensates by decreasing the number of oxytocin receptors to maintain body equilibrium (balance). So, prolonged exposure to Pitocin® in labor may lead to a reduction in the number of oxytocin receptors, called receptor desensitization or oxytocin receptor down-regulation. This is less likely to happen in physiologic labor and birth (with the body’s own oxytocin) because the oxytocin is released in pulses and then rapidly broken down by an enzyme so that there is very little (if any) oxytocin left in the bloodstream between pulses (Fuchs & Fuchs, 1984). Getting that break from oxytocin exposure protects against receptor desensitization, helping to keep contractions effective.
What Is Postpartum Hemorrhage?
Postpartum hemorrhage (PPH) is bleeding too much after birth. When PPH occurs in the first 24 hours after birth, it is sometimes called primary or early PPH (ACOG, 2017). When hemorrhage occurs from day two up until 12 weeks after birth, it is called secondary, late, or delayed PPH. This Evidence Based Birth® article focuses on primary PPH.
It is helpful to remember “the 4 Ts” that can cause postpartum hemorrhage (ACOG, 2017):
Tone
Loss of muscle tone in the uterine muscle is also called uterine atony. Atony literally means “without tone.” Uterine atony is a serious condition responsible for 70%-80% of all PPH. Since it is the most common cause of PPH, care providers should suspect it first, before the other possible causes. If the birthing person’s uterus lacks tone, that means it is not able to contract enough after birth to stop blood loss. The reason Pitocin® is given during the third stage of labor in active management is to help ensure the uterus will contract and have tone to prevent bleeding too much. In cases where tone is lacking, Pitocin® treats the atony and promotes uterine tone.
Induction of labor with Pitocin® and prolonged use of Pitocin® during labor are considered risk factors for uterine atony (ACOG, 2017; SOGC, 2018). This is because Pitocin® can cause more frequent, longer, and stronger contractions than the body’s own pulses of oxytocin, thus increasing the risk of uterine muscle exhaustion.
Labors that are prolonged or rapid (with very strong contractions) can also lead to uterine atony and increase the risk of PPH.
Other factors that can decrease tone in the uterus besides exhaustion of the uterine muscles include infection, having a large uterus (e.g., from high levels of amniotic fluid, a large baby, or multiples), having an unusually shaped uterus (e.g., from non-cancerous growths in the uterus called fibroids), or taking drugs that relax the uterus to prevent preterm labor (Association of Ontario Midwives, 2016).
Trauma
Trauma in birth can be physical or psychological. In this context, trauma refers to physical injury—such as uterine rupture; tears to the cervix, vagina, or perineum; and episiotomy—that can cause PPH. Tears must be repaired in order to stop the bleeding. Pitocin® after birth with active management does not directly reduce blood loss caused by tissue damage (these tears must be repaired to stop the bleeding), but it reduces overall blood loss after birth, which becomes more beneficial when someone is losing blood from injury.
Tissue
When portions of the placenta or membranes are left behind in the uterus they can prevent adequate contractions and contribute to PPH. The tissue must be removed to stop the bleeding. Having disorders of the placenta can put the birthing person at increased risk for leaving tissue behind (Association of Ontario Midwives, 2016). For example, placenta accreta is a serious condition where the placenta grows too deeply into the uterine wall, most often due to scarring from a prior Cesarean or other uterine surgery.
Thrombin
The “T” for thrombin reminds providers to evaluate if the mother is able to clot normally. If not, she may require treatment to help with blood clotting. This is thought to be the least common reason for PPH, causing about 1% of cases (Association of Ontario Midwives, 2016). Coagulation defects (clotting disorders) can cause a PPH or be the result of a PPH. They can be pre-existing (e.g., hemophilia), or acquired in pregnancy or labor (e.g., having low platelets, or clotting abnormalities with severe preeclampsia) (Evensen et al. 2017). Any of the previous three “T”s can become a clotting problem due to large losses of blood if left untreated long enough.
What is the Definition of Primary Postpartum Hemorrhage?
There is no standard definition of PPH that is used in the research and in professional guidelines around the world. Some amount of blood loss is normal and expected after childbirth. However, too much blood loss is a childbirth emergency that can be life threatening. The answer to the question of where to draw the line between normal, physiologic blood loss and excessive blood loss (i.e. postpartum hemorrhage) remains controversial.
Since the 1950s, PPH after vaginal birth has traditionally been defined as ≥500 mL blood loss within 24 hours of birth, and severe PPH has been considered ≥1000 mL blood loss (WHO, 2012). Until recently, this definition for PPH was applied to all birthing people regardless of their health status and whether or not they suffered any harmful effects from their blood loss after birth.
Postpartum hemorrhage is now being redefined based on research showing that healthy birthing people are usually unharmed by blood loss up to 1000 mLs (Anger et al. 2019). For perspective, blood loss around 500 mL is similar to a routine blood donation, a loss of about 2 cups of whole blood, which is usually well tolerated by healthy people. The blood that is lost after birth is sometimes also diluted with urine and amniotic fluid. In addition, there is an expansion of blood volume that occurs during pregnancy (by about 1250 mL) that helps to protect mothers against harmful effects of blood loss after birth (Erickson et al. 2017).
Harm from Blood Loss in the Third Stage of Labor Depends on the Individual
A review by Erickson et al. included two randomized trials where researchers randomly assigned (like flipping a coin) participants to expectant versus active management. None of the participants had received any Pitocin® to start or speed up labor. They found that the average blood loss (calculated by weight) was just over 500 mL among the people who did not receive Pitocin® after birth, and closer to 400 mL among those who received the Pitocin® with active management. So, it’s possible that blood loss around 500 mL could be considered normal or physiologic blood loss after childbirth. Accordingly, a joint consensus statement of midwifery organizations in the United States included “Results in physiologic blood loss” as one of the components of normal physiologic childbirth (ACNM, MANA, NACPM Consensus Statement, 2012).
On the other hand, it’s important to consider that individuals vary in when they will experience harm from blood loss. Some people experience signs and symptoms before losing 500 mL of blood and others can lose 1000 mL or more of blood after birth without clinical effects. Birthing people with anemia, low body mass, and those who have decreased blood volume (from dehydration or preeclampsia, for example) are more likely to feel the effects of losing even a few hundred mL of blood (Association of Ontario Midwives, 2016).
The U.S. reVITALize definition for early PPH accounts for the fact that the clinical consequences of blood loss vary by individual. The definition is formally endorsed by the American College of Obstetricians and Gynecologists (ACOG), the American Academy of Family Physicians (AAFP), the American College of Nurse-Midwives (ACNM), the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN), and the Society for Maternal-Fetal Medicine (SMFM).
They now define early PPH to be (1) blood loss of ≥1000 mL or (2) any amount of blood loss that is accompanied by signs/symptoms of hypovolemia (decreased blood volume) within 24 hours following a vaginal or Cesarean birth. Signs/symptoms of hypovolemia may include fast heartbeat, low blood pressure, fast breathing, low urine output, unhealthy paleness, dizziness, or altered mental state. When blood loss exceeds 500 mL after a vaginal birth or 1,000 mL after a Cesarean, it should trigger increased supervision, a rapid and thorough evaluation to identify the cause of the bleeding, and potential interventions as clinically indicated, but it does not make the diagnosis of PPH alone without additional signs/symptoms of decreased blood volume (ACOG, 2017).
How Do Care Providers Assess Blood Loss in the Third Stage of Labor?
Visually estimating how much blood has been lost is the most common method of determining blood loss volume after birth, where the care provider looks at the amount of blood lost and tries to estimate its volume (Diaz et al. 2018). Research has consistently shown that care providers tend to over- or under-estimate blood loss volume with their visual estimates. The same holds true across provider types and birth settings. Smaller volumes of blood loss tend to be overestimated and larger volumes of blood loss tend to be underestimated (Hancock et al. 2015). One study author made the observation that “estimates simply reflected teaching about what constitutes average blood loss at birth” (Razvi et al. 1996). So, providers might see what they expect to see based on their training about typical blood loss after birth. There are conflicting findings on whether years of experience and additional training can improve visual estimation skills.
A more precise method of assessing blood loss volume is using direct blood collection and measurement, often referred to as “quantified blood loss.” With this method, all of the blood that is lost in the third stage of labor (except the placenta and membranes) is funneled out from under the birthing person’s buttocks and into a collection device such as a bag, a bedpan, or an absorbent pad, where the blood can be measured.
When blood loss is actually measured rather than visually estimated, there is less potential for introducing bias from the diagnosing clinician. Racial bias (i.e., racism) in PPH assessment is a major concern, because poor recognition of PPH delays treatment. A large study with over 360,000 participants with PPH in U.S. hospitals found that non-Hispanic Black birthing people were at the highest risk for severe illness from PPH, and at five times higher risk for death compared to non-Hispanic White birthing people (Gyamfi-Bannerman et al. 2018).
Quality improvement projects to reduce PPH usually include quantified blood loss as part of a larger safety bundle to improve readiness, recognition and response to PPH. In this way, using quantified blood loss helps improve communication between providers. A recent study on a large quality collaborative in California found that hospitals using the PPH safety bundle with support from outside mentors were able to reduce severe maternal complications from PPH (Main et al. 2017).
Although quantified blood loss is a more accurate and objective way to assess blood loss volume, it may not be practical in all birth settings. And currently, there is no evidence from randomized trials that measuring blood loss results in better clinical outcomes compared to visually estimating blood loss. A Cochrane review found only two trials evaluating methods for estimating blood loss after vaginal birth (Diaz et al. 2018). There wasn’t enough evidence to recommend direct measurement of blood loss over visual estimation. However, the promising findings from the large quality improvement collaborative in California suggest quantified blood loss may offer benefits over visual estimation.
In addition to blood loss volume, care providers also assess the speed of blood flow and the birthing person’s response to the blood loss, including their pulse and blood pressure (Hancock et al. 2015). A review of the research concluded that these are also important factors in early diagnosis and treatment of PPH.
How Common Is Primary Postpartum Hemorrhage?
Estimates vary widely, depending on which definition of PPH is used. Randomized trials have found rates of blood loss >1000 mL ranging from 0.9%-2.9% with a routine uterotonic given in the third stage of labor versus 2.4%-4.8% without a routine uterotonic (Begley et al. 2019; Salati et al. 2019).
In the United States (U.S.), the rate of PPH >500 mL after vaginal birth (or 1000 mL after Cesarean birth) was 3% in a large study of 8.5 million hospital births between 1999 and 2008 (Kramer et al. 2013). During the study period, the overall rate of PPH stayed around 3%. However, the rate of severe PPH that led to blood transfusion, surgical removal of the uterus, or surgical repair of the uterus rose from 1.9 to 4.2 per 1000 from 1999 to 2008.
A recent study from the Netherlands looked specifically at PPH >1000 mL among 2.5 million people who gave birth between 2000 and 2013 (Van Stralen et. al 2016). They found that PPH rose significantly from 4.1% in 2000 to 6.4% in 2013.
Why is the Rate of Postpartum Hemorrhage Rising in Recent Years?
Australia, Canada, the U.K., and the U.S. have all reported a recent increase in the rate of PPH, and researchers aren’t sure why (Kramer et al. 2011). They speculate that it may relate to an increase in mothers with risk factors for PPH: more people giving birth after Cesarean, more multiple pregnancies, and an increase in induction, augmentation, and epidural use. However, there is very limited evidence to support these possible explanations.
Researchers in Canada looked back at over 100,000 births to single babies between 1978 and 2007 to try and explain their recent rise in PPH (Kramer et al. 2011). All of the births took place at a single hospital in Montreal. Hemorrhage was defined as >500 mL for vaginal births and >1000 mL for Cesarean births. Blood loss was based on the obstetrician’s visual estimates and management approach varied (active versus expectant).
Overall, 2.3% of the participants had a PPH during the study period, and the rate rose between 1978 and 2007. In addition to the increase in PPH, there was also an increase in labor induction, labor augmentation, and prior Cesarean section over the study period. The researchers concluded that these three risk factors (induction, augmentation, and prior Cesarean) appeared to largely explain the increase in PPH.
However, another study from Australia was not able to explain their rise in PPH by taking risk factors into account. PPH was defined as >500 mL after a vaginal birth or 750 mL after a Cesarean birth. Again, blood loss was based on visual estimates, and provider management approaches varied. Out of over 700,000 births between 1994 to 2002, the rate of PPH increased even after considering the birthing person’s age, prior Cesarean section, multiple pregnancies, induction/ augmentation of labor, and epidural use (Ford et al. 2007).
More research is needed, using a standardized definition for early PPH, because we do not yet have solid evidence on why the rate of PPH is increasing.
What Factors Increase the Risk of Postpartum Hemorrhage?
First, it’s important to point out that anyone can experience excessive blood loss after birth, even if they have no known risk factors for PPH. This is why many professional organizations recommend active management with the routine use of Pitocin® for everyone, regardless of individual risk factors for PPH.
However, studies have identified many factors linked to increased risk of blood loss ≥1,000 mL. It may be useful to discuss these risk factors with your care provider and consider how they apply to your unique situation when deciding between active and expectant management for the third stage of labor. Some of these risk factors can be known before the birth, and some can only be known after the birth.
Table 1 lists risk factors for postpartum hemorrhage defined as blood loss ≥1000mL and/or blood transfusion, surgical repair, or surgical removal of the uterus. The data come from population studies in the U.S., the Netherlands, Canada, Norway, Israel, and Sweden (Association of Ontario Midwives, 2016, Table 2, Page 9; Sheiner et al. 2005; Kramer et al. 2013; van Stralen et al. 2016; Oberg et al. 2014).
Table 1. Factors Linked to Increased Risk of Blood Loss ≥1,000 mL

What Is the Normal Length of the Third Stage of Labor?
Providers are careful to monitor the time that passes after the birth and before the placenta is expelled because a longer third stage of labor is linked to increased risk of PPH. When the placenta is not expelled after birth and remains in the uterus, it is diagnosed as a retained placenta and is often manually removed. Manual removal of the placenta is an invasive procedure that can be painful. The care provider puts on a sterile glove and inserts a hand through the cervix and into the uterus. The provider’s hand moves in a careful up and down motion to sweep behind the placenta and manually separate it from the wall of the uterus.
There are different professional opinions about what length of the third stage of labor is “normal” versus a third stage of labor that is abnormally long and would benefit from intervention.
A large study with nearly 13,000 single vaginal births found that, on average, 50% of placentas were birthed within 6 minutes and 97% were birthed by 30 minutes (Combs and Laros, 1991). In this study, most of the birthing people received active management of the third stage of labor. Factors linked to a longer third stage of labor in this study included preterm birth (a major risk factor), giving birth lying in bed (as opposed to upright), preeclampsia, augmented labor, and being a first-time mother.
In a review that looked at people who had expectant management of the third stage of labor, researchers concluded that it takes longer to birth the placenta with expectant management: 80% of mothers birthed their placenta by 30 minutes and 98% by 60 minutes (Weeks, 2008).
Recent meta-analyses of randomized trials (multiple trials combined into one large study) have not found a significant difference in the length of the third stage of labor with active versus expectant management (Begley et al. 2019; Salati et al. 2019). These meta-analyses concluded that there is low to very low–quality evidence that active management or Pitocin® alone may make little or no difference to the average length of the third stage of labor. However, earlier data (mentioned above) suggest that the third stage of labor may be longer with expectant management.
UpToDate® guidance for clinicians recommends that providers take into account how the third stage of labor is managed when they are deciding when to offer intervention in the third stage of labor. They say that the waiting period can be extended for expectantly managed third stages of labor and that “waiting as long as 60 minutes [with term births] is reasonable if an intervention can be promptly and successfully initiated if the patient begins to bleed” (Weeks et al. 2019).
In the U.K., the National Institute for Health and Care Excellence (NICE) guidelines define a prolonged third stage of labor as not completed within 60 minutes of the birth after expectant management and within 30 minutes of the birth with active management (NICE, 2019). They recommend a change from expectant to active management if the birthing person has PPH or the placenta is not expelled within one hour of the birth of the baby.
What Is the Evidence on Management of the Third Stage of Labor?
Randomized Trials Comparing the Active Management Package to the Expectant Management Package
A recent Cochrane review examined four randomized trials with nearly 5,000 participants who were assigned to active versus expectant management in hospital settings (Begley et al. 2019). Three of the studies were from the United Kingdom and one was from Ireland.
It is worth noting that the care providers who attended the births in these studies were more familiar with active management than expectant management. Some of the participants were considered at low risk for excessive bleeding after birth and some were at higher risk (i.e. had more risk factors for bleeding). Many of the participants in these studies had their labors induced or augmented.
What did they find?
Benefits of Active Management of the Third Stage of Labor
Overall, there was high-quality evidence that active management reduced the average amount of blood loss at birth (by around 80 mL).
It’s important to note, however, that only one of the four studies actually measured blood loss (the rest estimated it), and are therefore open to mistakes in measurement as well as possible bias from lack of blinding. In other words, care providers were not blinded to participants’ group assignments (active versus expectant) and may have assessed blood loss based on their beliefs about which birthing people were at greater risk of losing more blood.
Since visual estimations of blood loss can be subjective and unconsciously affected by a person’s beliefs, the Cochrane authors also reported the birthing person’s hemoglobin (Hb) less than 9 g/dL at 24 to 72 hours as a hard outcome relating to blood loss. They found that fewer mothers had anemia after birth with active versus expectant management (4% versus 7%; low-quality evidence). However, they point out that the average difference between groups may not be clinically important, as routine blood donation reduces Hb levels by a similar amount without ill effects in healthy women, and levels may rise within a few days after birth.
Active management reduced blood loss greater than 500 mL at birth (5% of participants lost >500 mL with active versus 15% with expectant management; moderate-quality evidence).
As far as blood loss ≥1,000 mL at birth, active management also showed benefits; however, the Cochrane authors called this finding “uncertain” because the evidence was considered very low-quality. The absolute risk of blood loss ≥1,000 mL at birth was 0.9% of people with active versus 2.4% of people with expectant management (three randomized trials). To put it another way, there would be one fewer PPH ≥1,000 mL after birth for every 66 people who had active management.
On a side note, research on planned home birth in the U.S.—where expectant management is routine care—has found that a similar number of people are estimated to lose greater than 500 mL of blood (15.5%) and greater than 1000 mL of blood (4.8%) (Cheyney et al., 2014).
In the Cochrane review, fewer participants assigned to active management in the trials required a blood transfusion (1% with active versus 3% with expectant management; low-quality evidence), and far fewer birthing people received therapeutic (or “rescue”) uterotonic medications within 24 hours of the birth (4% versus 21%; moderate-quality evidence).
They found no difference in the number of babies admitted to newborn care units or the number of babies with jaundice requiring treatment between the participants who received active versus expectant management. There was also no evidence that active management makes a difference in the need to remove the placenta manually or the average length of the third stage of labor.
Downsides of Active Management of the Third Stage of Labor
In addition to the benefits for blood loss prevention, there were also some downsides to active management for mothers and babies (low-quality evidence). Birthing people who received active management had an increase in blood pressure (3% versus 1%); however, this was probably due to ergometrine-containing preparations, not Pitocin®. With active management there was more vomiting after birth (7% versus 3%); more afterpains requiring pain medication (5% versus 2%); and a higher rate of return to hospital for bleeding (3% versus 1%).
The increase in the number of people returning to the hospital with bleeding is concerning—for every 65 people who had active management, one returned to the hospital because of bleeding. The authors think that the increased risk of late bleeding might have been due to shreds of tissue left behind after cord traction.
Not surprisingly, active management reduced the average birth weight of the baby (by about 80 grams; moderate-quality evidence) due to lower blood volume from early cord clamping. This effect could probably be avoided by changing the active management approach to include delayed cord clamping (waiting until the cord stops pulsating).
None of the included studies reported any maternal or newborn deaths.
The Cochrane authors looked separately at the risk of blood loss ≥1,000 mL in two trials with nearly 3,000 participants considered to be at low risk of PPH. These two studies varied in their specific criteria for “low-risk.” Both of them excluded birthing people for a history of hemorrhage; having given birth >5 times; anemia during pregnancy; a placenta that covered the cervix or separated from the uterine wall before birth; forceps or vacuum use; epidural use; and medical conditions that could increase their risk of bleeding after birth, such as heart disease or high blood pressure. All of the participants in the studies were planning to give birth vaginally to single, head-down babies. They found no evidence that active management lowered the risk of blood loss ≥1,000 mL in people at low risk for bleeding.
None of the studies looked specifically at people at high risk for bleeding after birth.
Conclusion
In conclusion, the Begley et al. (2019) Cochrane review found both benefits and harms to active management. Active management reduced the risk of severe blood loss and anemia after birth in a group that included both people at lower risk for bleeding and those at higher risk for bleeding. However, it also increased the need for pain medication for afterpains and increased the risk of excess bleeding following hospital discharge.
It is possible that the benefits of reduced blood loss can be achieved with less harm by making changes to the active management approach. These changes could include using a uterotonic medication that does not raise blood pressure (such as Pitocin®), improving skill or eliminating cord traction, and delaying cord clamping.
The authors recommend that pregnant people be given information on both the potential benefits and harms of active management to support informed choice. Unfortunately, the evidence on which management approach reduces the risk of severe blood loss >1,000 mL is very low quality, so it’s difficult to draw firm conclusions, especially among people at low risk of PPH. The quality of the evidence is higher showing that active management reduces average blood loss (by around 80 mL) and the rate of blood loss >500 mL, but these outcomes are not as clinically important as a reduction in blood loss ≥1,000 mL. If someone chooses expectant management, it is important that they have a uterotonic drug available if excess bleeding occurs.
Randomized Trials Comparing the Active Management Package to Different Mixed Management Packages
The Begley et al. (2019) Cochrane review also looked at four trials of active versus mixed management of the third stage of labor. Unfortunately, all of the mixed managements differed, so they were not able to draw firm conclusions on any of the approaches.
Out of the four trials on mixed management, only one was considered to be high quality. This study included healthy pregnant people in Sweden between 34 and 43 weeks with single, head-down babies (Jangsten et al. 2011). None of the participants had experienced a previous PPH, had preeclampsia, or had given birth >5 times. However, the study did include people with risk factors for PPH. About 4% of each group had a prior Cesarean, 40% had an epidural, and 50% had their labors sped up with synthetic oxytocin.
About 800 people were randomly assigned to active management and 800 people were assigned to mixed management. The active management approach consisted of giving synthetic oxytocin within 2 minutes of birth, immediate cord clamping, controlled cord traction while the birthing person pushed, and uterine massage after birthing the placenta. In comparison, the participants assigned to mixed management received a placebo of IV saline solution instead of the synthetic oxytocin. They had the cord clamped immediately, but did not receive any cord traction during pushing. Again, everyone received uterine massage after the placenta was birthed. All blood loss up to two hours after birth was collected and measured.
Compared with mixed management that used early cord clamping and no uterotonics or cord traction, active management with a uterotonic reduced the use of therapeutic uterotonics within 24 hours of the birth (5% versus 18%). Active management also reduced blood loss of 1,000 mL or more up to two hours after birth (10% versus 17% – surprisingly high rates!), it reduced the length of the third stage by a couple of minutes, and it increased the mother’s average Hb levels after birth. This study found that active management was linked to lower pain scores at two hours after birth compared to mixed management without a uterotonic.
Randomized Trials Comparing Pitocin® Versus No Pitocin®
What is the evidence on using Pitocin® versus no Pitocin®, regardless of the other parts of the active management package?
Salati et al. (2019) examined nine trials that randomly assigned participants to routine Pitocin® in the third stage versus no uterotonic drug or placebo. The intervention in these trials could be considered “mixed management” because it included Pitocin® but varied in the other components of active management. For example, the studies differed in whether or not they used controlled cord traction or immediate cord clamping. Most of the births took place in freestanding birth centers or hospitals, although one trial from the Netherlands included home births. The studies came from France, Germany, the Netherlands, Sweden, South Africa, Tunisia, and the U.K.
Like Begley et al. (2019), they found that Pitocin® reduced the risk of blood loss >500 mL and >1000 mL after birth (low quality evidence). The absolute risk of blood loss >1000 mL was 2.9% of people with synthetic oxytocin versus 4.8% without (five randomized trials). They also found moderate quality evidence that it reduced the use of additional uterotonics as treatment for PPH.
Again, there was no evidence that active management made a difference in the need to remove the placenta manually or the length of the third stage.
Unlike Begley et al. 2019, they found no difference in the risk of receiving a blood transfusion between the birthing people assigned to Pitocin® versus no uterotonics or placebo. Salati et al. did not examine delayed blood loss (>24 hours to 6 weeks) after birth, so we don’t know how that factor compared between groups.
Observational Studies Comparing Active Versus Expectant Management
As we mentioned earlier, the evidence we have from randomized trials comes from settings where the care providers are more familiar with active than expectant management, and where labor interventions, including synthetic oxytocin to augment labor, are extremely common. Researchers have called for more studies to be conducted in areas where providers are familiar with supporting the physiologic third stage, such as the Netherlands and New Zealand, and in settings where routine labor interventions are not the norm (Begley et al. 2019).
We found four retrospective studies (where researchers look back in time at birth outcomes) in the last 10 years that reported rates of PPH and the type of management that was used during the third stage (Table 2). We were looking for studies that reported the rate of PPH among people who received active management and the rate of PPH among a similar group of people who had expectant management. The studies took place in Ireland (Dencker et al. 2017), England (Homer et al. 2017), New Zealand (Dixon et al. 2013), and Australia (Fahy et al. 2010).
Table 2. Four Retrospective Studies Reporting Rates of PPH and Approach to Third Stage (Expectant versus Active)


These are observational data, not from a randomized trial, so the evidence is lower quality. We can’t rule out the presence of factors that bias the results; for example, longer labors are linked to more PPH and more active management (Dixon et al. 2013).
Two of these studies examined PPH by type of third stage management as their primary outcome. They reported the rate of PPH >1,000 mL among low-risk pregnant people to be 0.5% to 3.4% with expectant management and 2.9% to 9.9% with active management.
The results from these observational studies show no increase in blood loss with expectant management. On the contrary, they found that people who had expectant management had a lower risk of PPH, and active management increased the risk of PPH.
The largest study included over 32,000 low-risk pregnant people in New Zealand who received midwife-led care in a variety of birth settings from 2004 to 2008 (Dixon et al. 2013). About half of the participants received expectant management and half received active management. In addition to higher levels of PPH >1,000 (9.9% vs. 3.4%), active management was also linked to more need for manual removal of the placenta (0.7% vs. 0.2%).
Also, they found that the people who had active management and then treatment for excessive bleeding had more blood loss of >500 mL than those who had expectant management and then treatment. This suggests that uterotonic drugs might provide more effective treatment for PPH when there has been no prior routine exposure (leading to receptor desensitization and/or uterine atony).
The quality of evidence from observational studies is not as high as randomized trials; still, these findings raise questions about whether the benefits of reduced blood loss seen in randomized trials apply to pregnant people at low risk for PPH who experience expectant management with a skilled provider after a physiologic birth.
Evidence on Best Practices to Use With Expectant Management in the Third Stage of Labor
Researchers interviewed expert midwives in Ireland (9 midwives) and New Zealand (18 midwives) about the skills they used for expectant management of the third stage of labor (Begley et al. 2012). To be included in the study, the midwives had to use expectant management with at least 30% of their births over the previous two years and have an average PPH rate (defined as >500 mL) with expectant management of less than 4% (which is a very low PPH rate with expectant management).
In general, the expert midwives expressed that their role during the third stage of labor was one of “watchful waiting” and “alert vigilance.” Expectant management was only considered appropriate following an uncomplicated labor without epidural or synthetic oxytocin for induction or augmentation. The interviews revealed that the midwives shared many common beliefs about how to best support the physiologic third stage of labor. Some of the important components of expectant management that were identified by these skilled midwives included:
- Carefully observe and listen to the birthing person, paying close attention to behavior and feelings:
- For example, if the mother describes cramping or a sore back, this alerts the midwife that the placenta may have separated; if the birthing person feels dizzy and unwell, this alerts the midwife to the threat of excess blood loss
- Watch for other signs of placental separation, which include a “normal separation bleed,” also described as a “single gush” followed by a lengthening of the cord
- Foster a birth environment that feels warm, calm, quiet, and safe
- Encourage mother-baby attachment by facilitating skin-to-skin contact immediately after birth
- Place the baby on the chest in order to encourage it to initiate its own feed, or proactively latch the baby if there is a concern about blood loss or a delayed placenta
- Do not rush to clamp the cord
- Use truly upright positions and maternal effort to birth the placenta, especially if there is a delay (walking and sitting on the toilet was used often)
- Offer the mother food or fluids to replace her energy for the effort required to birth the placenta
- Assist with gentle cord-traction by giving “a little lift” or “easing down and out” if the placenta has separated and descended into the vagina
- Plan ahead—discuss the birthing person’s risk factors for PPH and wishes for the birth; prepare in advance for complications
Some researchers argue that the definition of “expectant management” used in randomized trials is extremely limited and might as well be described as “not active management” (Hastie and Fahy, 2009). This narrow definition of expectant management leaves out most of the elements of physiologic third stage care that midwives believe are important, and therefore the findings from the randomized trials can not be generalized to midwife-led and community birth settings.
Hastie and Fahy propose an alternative to active management that they describe as a holistic approach called “psychophysiological” third stage care. It includes best practices based on scientific theory and midwife expertise. Basically, they explain how environmental factors in the birth environment (such as lack of privacy, bright lights, noise, the presence of strangers) may disrupt the protective hormonal changes that should occur immediately after birth. To optimize a birthing person’s psychophysiology, they say that the midwife must act as a guardian so that the mother and baby can experience an undisturbed third stage of labor. Read an interview with the authors here at Lamaze International!
Dr. Sarah Buckley, a New-Zealand-trained family physician and researcher in this field, explains that the fight-or-flight hormones adrenaline and noradrenaline (epinephrine and norepinephrine, also known as catecholamines) can help to keep the birthing person awake and energized to push in the second stage of labor (Buckley, 2020). However, in the third stage of labor, these levels start to fall, and if the mother is cold, shivering, worried, or distracted, continuing high levels of catecholamines can disrupt the beneficial effects of oxytocin.
The baby also requires warm skin-to-skin contact to lower their stress hormones and promote nipple-seeking behaviors. Skin-to-skin contact and breastfeeding/chestfeeding further increase oxytocin levels in both the mother and baby.
In summary, expectant management should be much more than simply doing nothing or “not active management.” Providers skilled at expectant management use many techniques to support the birthing person’s own naturally produced (endogenous) release of oxytocin.
What Is the Evidence on Breastfeeding/Chestfeeding or Nipple Stimulation to Prevent PPH?
A 2016 Cochrane review that included four randomized trials concluded that there is currently not enough evidence to know whether early suckling or nipple stimulation is effective for reducing bleeding during the third stage of labor (Abedi et al. 2016).
We found one randomized trial on this topic that was too recent to be included in the 2016 Cochrane review (Dashtinejad et al. 2018). This study randomly assigned 108 birthing people in Iran to breast stimulation or synthetic oxytocin in the third stage of labor. None of the participants had their labors induced or augmented. People in the stimulation group received breast pump stimulation (10 min for each breast with a negative pressure of 250 mmHg), while those in the synthetic oxytocin group received an infusion of 30 IU synthetic oxytocin in 1,000 mL of Ringer’s serum with a maximum rate of 10 mL infusion per min after birth.
There were no differences in the rate of blood loss >500 mL or anemia between the groups. In other words, breast stimulation worked as well as synthetic oxytocin to prevent bleeding in this study. Moderate to severe after-birth pain was significantly lower in the stimulation group compared to the synthetic oxytocin group (9% versus 74%) and more people in the stimulation group said they were highly satisfied with their care (85% versus 6%). The authors concluded that using breast stimulation is cost-effective and may be considered for women at low risk for PPH.
Interestingly, using nipple stimulation before birth to induce labor may help to prevent excess blood loss after birth. A Cochrane review combined two randomized trials from 1993 with 300 total participants and found significantly less PPH (not defined) after birth among those who had stimulated their nipples to induce labor (Kavanagh et al. 2005). The participants had stimulated one nipple at a time for one hour per day for three days. The rate of PPH was 0.7% with nipple stimulation to induce labor versus 6% with no stimulation.
Researchers have actually measured oxytocin levels in saliva before and after nipple stimulation to induce labor and demonstrated that oxytocin levels rise after the stimulation (Takahata et al. 2018). So, there is evidence that nipple stimulation supports the birthing person’s own oxytocin levels. However, we do not yet have research evidence that nipple stimulation or early suckling during the third stage of labor leads to fewer cases of blood loss >1,000 mL.
Evidence on Best Practices to Use With Active Management in the Third Stage of Labor
Which Is the Better Route to Use for Pitocin® in the Third Stage of Labor: Intramuscular (IM) or Intravenous (IV)?
There is disagreement between professional organizations about the best route of giving Pitocin® in the third stage of labor. Some recommend injection into a muscle (intramuscular or IM administration) as the preferred route (e.g., the Society of Obstetricians and Gynecologists of Canada, 2018) and others recommend both IM and injection into a vein (intravenous or IV administration) equally (e.g., the World Health Organization, 2018)
Recent guidelines from WHO, ACOG, AAFP, SOGC, and NICE all recommend 10 international units (IU) as the dosage for giving Pitocin® IM with active management (WHO, 2018; ACOG, 2017; Evensen et al. 2017; SOGC, 2018; NICE, 2019). In the guidelines we reviewed, the recommended dosage for giving Pitocin® as an IV bolus (given over 1 to 2 minutes) ranged from 5 to 10 IU. See the section below on Recent Practice Guidelines for specific recommendations.
A 2018 Cochrane review examined the evidence on IM versus IV Pitocin® for active management in the third stage of labor. They combined three randomized trials from 2014, 2015, and 2016 with over 1,300 participants assigned to IM versus IV Pitocin® (Oladapo et al. 2018). The studies were carried out in hospital settings in Turkey (two studies) and Thailand (one study) and included pregnant people with single, term pregnancies. None of the studies were blinded. They did not find any differences between the IV and IM routes of administration, but the quality of the evidence was considered “low or very low.”
Similarly, the Cochrane meta-analysis by Salati et al. (2019), that we discussed earlier compared the IM route (four trials) to the IV route (five trials) and did not find any differences between groups. All of those trials took place between 1974 and 2010.
So, we don’t yet have evidence from Cochrane meta-analyses that one route is preferable to the other route. However, three randomized trials have taken place in the last couple of years and ALL of them have found benefits to giving by Pitocin® by IV instead of IM.
One of the trials was conducted in Argentina, one was from Ireland, and one was from Egypt. The trials from Argentina and Ireland were double blind; participants assigned to 10 IU of Pitocin® by IV received that dose along with a matching placebo dose of saline solution IM, and those assigned to 10 IU of Pitocin® by IM received that dose along with a placebo dose of saline solution IV (Adnan et al. 2018; Durocher et al. 2019). Neither the birthing people nor the clinicians knew which injection was the placebo and which was the Pitocin®. Both trials measured blood loss rather than relying on subjective clinician estimates.
Neither of these double-blind trials found a difference in blood loss ≥500 mL between groups assigned to IV versus IM Pitocin® given within one minute of the birth. However, Adnan et al. (2018) found that the IV group (a bolus of 10 IU in 1 mL given over one minute) had fewer cases of severe PPH of ≥1,000 mL (4.6% versus 8.1%), less use of blood transfusion (1.5% versus 4.4%), and fewer mothers admitted to intensive care (1.7% versus 3.7%). They found a similar rate of side effects (4%-5%) such as nausea, vomiting, low blood pressure, and rapid heartbeat between birthing people assigned to IV bolus versus IM Pitocin®.
The other double-blind trial by Durocher et al. (2019) found that birthing people in the IV group (an infusion that took 40 minutes to complete on average) received fewer additional uterotonic medications compared to those in the IM group (5% versus 12%). The authors think the reason for this could be that people bleed more initially after IM injection (leading to more “rescue” uterotonics), since the IM Pitocin® takes longer to start working (3 to 7 minutes) compared to IV Pitocin®, which starts working right away. Rates of blood loss ≥500 mL were similar in both groups.
The third trial from Egypt was not blinded, but it did have several strengths (Charles et al. 2019). With nearly 5,000 participants, it was the largest of the three trials, and it excluded birthing people who received any synthetic oxytocin before the birth (such as to start or speed up labor). It also compared the IM route with two different ways of giving synthetic oxytocin—slowly as an IV infusion or quickly as an IV bolus. Like the other trials, this one also measured blood loss instead of estimating it.
This trial had three groups: The first group, assigned to the IM route, received 10 IU of synthetic oxytocin injected in the thigh. For the second group, which received an IV infusion, the synthetic oxytocin was mixed in 500 mL of fluid and given through gravity infusion with the roller clamp fully open, using an 18-gauge needle. The average time to complete the infusion was 28 minutes. The third group received an IV bolus; the bolus was pushed directly into the IV port over about one minute.
In this trial, average total blood loss was significantly less in both IV groups compared to the IM injection group. The risk of having blood loss ≥500 mL was less after IV infusion (0.8%) than after IM injection (1.5%). There was a trend toward lower risk of blood loss ≥500 mL after IV bolus (1.0%) compared to IM injection, though this finding was not statistically significant. There were no serious side effects (intensive care unit admission, shock, or death) in any of the groups.
Durocher et al. (2019) combined the data from these three trials in a meta-analysis and found that the IV routes, compared to the IM route, was linked to a significantly lower rate of PPH ≥1,000 mL (1.2% with IV versus 1.6% with IM).
In summary, the results from these three trials suggest that giving Pitocin® either as an IV bolus pushed over one minute or as an IV infusion completed in around 30 minutes may help to reduce blood loss after birth better than IM injection among people receiving active management of the third stage of labor. However, there are other factors to consider in deciding which route to use, such as birth setting, provider skill levels, available resources, and birthing people’s preferences (Durocher et al. 2019). If an IV line is already in place, then giving Pitocin® by the IV route might make the most sense, but the IM route may be preferable if there is no IV line.
When Is the Best Time to Give Pitocin® in the Third Stage of Labor?
There are different options for timing that providers can use when giving Pitocin® in the third stage of labor. For example, it can be given with the birth of the baby’s front shoulder, immediately after the baby is born but before the cord is clamped, right after the cord is clamped, or immediately after the placenta is birthed.
Sometimes care providers base their timing on what they find most practical. There is also a concern that, in theory, giving Pitocin® with the birth of the baby’s front shoulder could harm an undiagnosed twin (Association of Ontario Midwives, 2016). Waiting until after the baby is born to give Pitocin® gives the provider time to rule out the presence of a twin.
Another concern we’ve heard is that giving Pitocin® before the placenta has separated from the uterine wall could cause strong contractions that close the cervix, thus trapping the placenta in the uterus (i.e. a retained placenta). But there is currently no evidence that the timing of giving Pitocin® makes a difference in the risk of retained placenta. The uterotonic ergometrine, on the other hand, has been linked to a massive increase in retained placentas. Unlike Pitocin®, ergometrine causes a powerful, continuous contraction that quickly closes the cervix, increasing the risk of a trapped placenta (Weeks et al. 2019).
Another issue to consider is that giving Pitocin® before clamping the umbilical cord may expose the baby to Pitocin®. There is no reported evidence of any harmful effects on the baby from giving Pitocin® in the third stage of labor, but some parents may still wish to avoid this exposure (Soltani et al. 2010).
A 2010 Cochrane review and meta-analysis was the first study to look closely at the evidence on the timing of Pitocin® in the third stage of labor (Soltani et al. 2010). They combined the data from three trials with 1,671 participants who were randomly assigned to receive Pitocin® before giving birth to the placenta (with the birth of the baby’s front shoulder) or immediately after giving birth to the placenta.
Two of the trials were double blind, where neither the mothers nor their care providers knew who got the Pitocin® and who got a saline placebo. Two of the trials gave Pitocin® by IV infusion and one gave it by IM injection. All of the trials clamped the umbilical cord within about 30 seconds of the birth and all used controlled cord traction.
The researchers did not find any benefits from giving Pitocin® before versus after birthing the placenta. There was no difference in blood loss >500 mL or >1,000 mL, retained placenta, length of the third stage of labor, changes in hemoglobin, blood transfusion, the use of additional uterotonic drugs, or the rate of low blood pressure.
There is surprisingly little research on the timing of Pitocin® in the third stage of labor. We found one randomized trial from Turkey that was too recent to be included in the Cochrane meta-analysis (Oguz Orhan et al. 2014). This trial randomly assigned pregnant people with a single baby at term to one of four groups: IV Pitocin® with the birth of the baby’s front shoulder, IV Pitocin® immediately after the baby was born, IM Pitocin® with the birth of the baby’s front shoulder, or IM Pitocin® immediately after the baby was born. There were 150 people in each group.
There were no differences between groups in blood loss after birth (which was measured) or the use of additional uterotonic drugs. The people assigned to IV Pitocin® with the birth of the baby’s front shoulder had the shortest third stage of labor on average compared to the other groups and they had the least reduction in hemoglobin and hematocrit levels (indicating anemia) after birth.
However, the authors think these effects are “clinically insignificant” and they say that they did not find evidence that the route or timing of Pitocin® impacts blood loss.
A 2013 Cochrane review and meta-analysis looked specifically at the timing of umbilical cord clamping and found that waiting to give Pitocin® until after delayed cord clamping does not increase the risk of PPH (McDonald et al. 2013). It included 15 randomized trials with nearly 4,000 mother-baby pairs assigned to early cord clamping, defined as clamping within 60 seconds of the birth, or delayed cord clamping, defined as clamping anywhere from after one minute of the birth to when the cord stopped pulsating.
They concluded that delayed cord clamping offers significant advantages to the baby, such as higher birth weight, increased hemoglobin levels at birth, and increased iron stores in the first several months of life, with no additional risk of PPH or retained placenta for the birthing person. The overall rates of jaundice were not different between the groups. Fewer babies assigned to early clamping received light therapy for jaundice (2.7% versus 4.4%); however, the data that found this association came from an unpublished dissertation study that didn’t have objective measurement of jaundice. A different meta-analysis published in the Journal of the American Medical Association (JAMA) did not include this one questionable study, and they did not find any relationship between jaundice and delayed cord clamping (Hutton & Hassan, 2007).
The Cochrane authors concluded that the potential benefits of delayed cord clamping outweigh the potential risks.
So, there is currently no evidence showing that the timing of routinely giving Pitocin® in the third stage of labor influences the rate of PPH. Waiting to give Pitocin® until after delayed cord clamping has not been found to increase the risk of PPH.
UpToDate® guidance for clinicians recommends giving Pitocin® after the birth of the baby’s front shoulder (before the birth of the placenta) plus using controlled cord traction after delayed cord clamping (Funai et al. 2019; Berghella et al. 2019). Pitocin® should not be given before the front shoulder is born as this could worsen a shoulder dystocia (a complication that occurs when the baby’s shoulders become stuck in the mother’s pelvis), if present.
The route of giving Pitocin® (IV or IM) affects how quickly the medicine starts to work. When it is given as an IM injection, it takes around 3 to 7 minutes to start working, whereas the effect is almost immediate when given by IV (Durocher et al. 2019). There is growing evidence that giving Pitocin® with the IV route may help to prevent blood loss better than the IM route, perhaps because of the quicker onset of action with IV.

Note in this photo above, the cord is white, limp, and not pulsating.
What Is the Evidence on Using Controlled Cord Traction With Active Management?
As we’ve discussed, the traditional active management approach involves giving the birthing person a uterotonic to help the uterus contract, clamping the baby’s cord early, and pulling on the cord while applying counter pressure to help pull out the placenta, called controlled cord traction (CCT). It is thought that CCT helps to shorten the length of the third stage of labor and reduce blood loss.
There is a concern that giving a uterotonic drug might cause strong contractions and lead to a retained placenta if the placenta is not pulled out quickly with CCT.
A Cochrane review of three randomized trials evaluated the effects of CCT on the third stage (Hofmeyr et al. 2015). Blinding was not possible in these studies, but measuring blood loss instead of relying on subjective estimates helped to minimize bias. They considered the evidence to be high quality.
We’re going to focus on the participants who were assigned to CCT or no CCT after receiving routine synthetic oxytocin (excluding those who received routine ergometrine as their uterotonic drug).
Out of 23,000 participants, there was no difference in PPH (≥500 mL), severe PPH (≥1000 mL), blood transfusion, or maternal death with or without controlled cord traction. There was a reduction in manual removal of the placenta with CCT when participants receiving ergometrine were included but not when the analysis restricted to people receiving synthetic oxytocin.
Controlled cord traction reduced blood loss by about 8 mL, which is not clinically significant, and reduced the length of the third stage by a minute or so. Fewer additional (“rescue”) uterotonics were used with CCT.
The authors conclude that “women who prefer a less interventional approach to management of the third stage of labor can be reassured that when a uterotonic agent is used, routine use of CCT can be omitted from the ‘active management’ package without a significant increase in risk of severe postpartum haemorrhage.” However, CCT may decrease the risk of manual removal of the placenta when ergometrine is used and when a strict 30-minute time limit is imposed on the third stage.
There is no research on the benefits and risks of CCT when a uterotonic drug is not used.
It’s important to mention that CCT requires skill for it to be performed appropriately, since it can result in serious complications when done incorrectly (Hofmeyr et al. 2015). If traction is applied before the uterus has contracted enough, and without applying effective counter pressure to the uterus, the uterus can be pulled inside out, called uterine inversion. Cord avulsion (cord “snapping” or cord rupture) is another potential complication that occurs in about 3% of births with CCT (Berghella, 2007). Lastly, as we mentioned, there is the potential for shreds of tissue to be left behind in the uterine cavity with CCT that could increase the risk of late bleeding.
Are There Benefits to Uterine Massage in the Third Stage of Labor?
Uterine massage involves placing a hand on the birthing person’s lower abdomen and stimulating the uterus to contract by rubbing and squeezing. (Massage is really a misnomer, since the forceful rubbing can be quite painful!)
A Cochrane review included two randomized trials with people who were randomly assigned to receive uterine massage or no massage after birthing the placenta (Hofmeyr et al. 2013).
The first trial included 200 people who were randomly assigned to uterine massage or no uterine massage after active management with routine synthetic oxytocin. Uterine massage was done every 10 minutes for one hour. There was no difference in blood loss >500 mL, but the average blood loss was about 40 mL less in the uterine massage group at 30 minutes and 80 mL less at 60 minutes. The use of additional uterotonics to treat heavy bleeding was less in the uterine massage group. No one experienced a retained placenta in either group.
A second and much larger trial assigned 2,000 birthing people to receive synthetic oxytocin, uterine massage or both. The group that received uterine massage alone experienced the most cases of bleeding >500 mL in 30 minutes. When synthetic oxytocin was used, they found no additional benefit from uterine massage.
We found one large randomized trial that came out after the Cochrane review (Chen et al. 2013). Here they randomly assigned 1,170 people to 10 units of synthetic oxytocin intramuscularly immediately after the birth of the baby’s shoulder plus 30 minutes of continuous uterine massage and 1,170 people to intramuscular synthetic oxytocin alone. The uterine massage in addition to synthetic oxytocin did not reduce blood loss compared to synthetic oxytocin alone.
In the uterine massage group, 378 (32.3%) people reported pain when receiving uterine massage and 16 (1.4%) people asked to stop the massage because of pain.
The Cochrane authors expressed a need for more research on the effectiveness of uterine massage versus no uterine massage with expectant management, since all of this research is on active management.
We’ve heard care providers say that they give gentle massage (without any real force) with expectant management to help the uterus shrink after birth. Either they give the gentle massage or they instruct the birthing person in how to massage their own uterus. However, we don’t have evidence yet from randomized trials to support using uterine massage with expectant management.
As far as active management, the largest study on this topic found no benefits to uterine massage when synthetic oxytocin was used. Furthermore, many women have contacted us at Evidence Based Birth® to state that the experience is extremely painful, and some have traumatic memories of this experience.
Uterine massage is distinct from postpartum assessment of fundal tone, which is recommended to identify uterine atony (WHO, 2012). To assess fundal tone, the care provider places their hand lightly on top of the uterus to feel whether or not it’s contracting regularly. If it’s not, they may ask the birthing person to either chestfeed/breastfeed the baby or self-perform nipple stimulation to encourage contractions by stimulating the body’s own naturally produced (endogenous) oxytocin.
Are There Any Trainings Health Care Professionals Can Take to Improve Outcomes With PPH?
Care providers can prepare for obstetric emergencies before they occur! The American Academy of Family Physicians (AAFP) designed a comprehensive, evidence-based course called Advanced Life Support in Obstetrics (ALSO™) training to prepare maternity care providers to effectively manage potential emergencies during pregnancy and childbirth, including PPH (Personal correspondence, Ann Evensen, MD, FAAFP, May 17, 2020).
In the ALSO training, learners complete an online prep portion of the course prior to attending a one-day in-person course. The in-person training is focused on hands-on demonstration of skills as well as team building and the importance of the team in managing obstetric emergencies. The course is taught by certified ALSO faculty who are ALSO providers and have completed an in-person course on ALSO instruction. They have to maintain certain requirements for certification, and they maintain a low instructor to student ratio. Over 100,000 care providers around the world have attended this course!
A 2011 study of around 1,000 births in Tanzania compared PPH outcomes before versus after providers completed the ALSO training (Sorensen et al. 2011). After the training, severe PPH (blood loss ≥1000 mL) was significantly reduced from 9.2% to 4.3%. So, completing the ALSO course is an evidence-based way for providers to reduce PPH.
The full ALSO course is important for all providers who regularly attend births regardless of birth setting. Even if someone does not have all of the medications and other interventions available to them in a home or freestanding birth center, the course will help them understand what is available and why the intervention might be used in an emergency. In addition, some of the maneuvers/interventions for PPH as well as shoulder dystocia (when the baby’s shoulder gets stuck during birth), breech birth, maternal resuscitation, and cord prolapse can be done in the community setting.
The Basic Life Support in Obstetrics (BLSO™) course is adapted from ALSO. It’s a good option for those who do not regularly attend births but want to be proficient in emergency assessment and management during childbirth. BLSO is designed to train first responders and emergency personnel and it’s an excellent introduction for students. We strongly encourage health care professionals and students to take advantage of ALSO or BLSO trainings!
Recent Practice Guidelines
The World Health Organization (WHO) recommends using Pitocin® (10 IU, IM/IV) with all births during the third stage of labor to prevent PPH (WHO, 2018). In settings where synthetic oxytocin is not available, they recommend using another effective uterotonic drug. The WHO considers the use of a uterotonic drug (i.e., Pitocin®) to be the most important part of the active management package (WHO, 2019). They say that controlled cord traction in combination with Pitocin® may add a small benefit when performed by a skilled attendant, but uterine massage probably adds no benefit to preventing PPH. The WHO recommends that the umbilical cord not be clamped any earlier than is necessary for controlled cord traction, which they say is normally around three minutes.
The International Confederation of Midwives describes the third stage of labor as a “time of adjustment” when the birthing person is adjusting to the changes that follow birth and the baby is adjusting to life outside of the uterus (2008, Revised 2017). It is their position that competency in both active and expectant management of the third stage of labor is “a basic midwifery competency.” The role of the midwife is to provide all of the necessary information and then support the birthing person’s informed decision about which interventions they desire for their “placental birth.”
The American College of Obstetricians and Gynecologists (2017, Reaffirmed 2019) recommends that all birth facilities should have guidelines in place for giving routine Pitocin® after birth. They say that Pitocin® can be given by IV infusion (bolus dose of 10 units), or IM injection (10 IU). ACOG states that “delaying oxytocin until after delayed umbilical cord clamping has not been found to increase risk of hemorrhage.”
The California Maternal Quality Care Collaborative (CMQCC) developed the OB Hemorrhage Toolkit V 2.0 in 2015 (CMQCC, OB Hem Task Force, 2015). They support using active management for all birthing people. However, they emphasize synthetic oxytocin as the main component of active management and clearly state that active management should not interfere with delayed cord clamping. Similarly, active management should not delay skin-to-skin care.
The Society of Obstetricians and Gynecologists of Canada (SOGC) recommends offering active management to everyone to reduce PPH (SOGC, 2018). They specify that 10 IU of synthetic oxytocin should be given IM with low-risk vaginal births, immediately after the birth of the anterior (front) shoulder. They consider an IV infusion of synthetic oxytocin (20 to 40 IU in 1,000 mL, 150 mL per hour) to be an acceptable alternative. An IV bolus of 5 to 10 IU (given over 1 to 2 minutes) can also be used after vaginal births.
The U.K.’s National Institute for Health and Care Excellence (NICE) defines active management as the routine use of uterotonic drugs, delayed clamping and cutting of the cord, and controlled cord traction after signs that the placenta has separated. They define ‘physiologic management’ as no routine use of uterotonic drugs, no clamping of the cord until pulsation has stopped, and birthing the placenta by the mother’s pushing efforts alone. They recommend active management for everyone, but say that a woman at low risk of PPH who chooses physiologic management should be supported in her choice. They recommend 10 IU of synthetic oxytocin by IM injection with the birth of the baby’s front shoulder or immediately after the birth of the baby and before the cord is clamped and cut.
The Association of Ontario Midwives published an excellent, very comprehensive guideline on PPH in 2016 (Association of Ontario Midwives, 2016). They recommend that midwives should identify risk factors for PPH during pregnancy and birth and discuss these risk factors and options for management of the third stage of labor in an informed choice discussion.
- If the birthing person chooses active management, midwives should use synthetic oxytocin; wait until pulsation stops to clamp and cut the cord; and use controlled cord traction to birth the placenta. Uterine massage is not recommended.
- If the birthing person chooses expectant management, midwives should watch for signs that the placenta has separated and monitor blood loss; wait until pulsation stops or the placenta is birthed to clamp and cut the cord; wait for the placenta to be born with maternal effort or gravity (skilled midwives may offer controlled cord traction); and encourage immediate skin-to-skin contact with the infant, early breastfeeding/chestfeeding, and other measures that may encourage the release of the mother’s natural oxytocin. Uterine massage is not recommended.
Conclusions
- Parents today can choose from three different types of management of the third stage of labor:
- Expectant management involves supporting the birthing person’s natural release of oxytocin, waiting to clamp the cord until it has stopped pulsating, and using gravity or the mother’s own pushing efforts to birth the placenta.
- Traditional active management is the routine use of a uterotonic medication, early cord clamping, controlled cord traction (pulling on the cord) to birth the placenta, and sometimes uterine massage. Randomized trials comparing active management with and without early cord clamping, controlled cord traction, and uterine massage have shown that the uterotonic medication is the most important part of the active management package.
- Mixed management combines methods from the expectant and active management approaches; for example, someone may choose physiologic (delayed) cord clamping and a uterotonic drug with no cord traction.
- Early cord clamping has traditionally been part of the active management package, but professional organizations around the world now discourage early cord clamping and no longer consider it part of an effective active management approach.
- Early cord clamping has been shown by randomized trials to cause harm to infants by lowering their iron stores and increasing the risk of iron-deficiency anemia.
- Parents can ask their care provider to leave the cord unclamped until it has stopped pulsating (usually takes at least 5 minutes), regardless of whether or not they use Pitocin® in the third stage of labor. Waiting to give Pitocin® until after delayed cord clamping does not increase the risk of PPH. During delayed cord clamping, the baby should be skin-to-skin on the birthing person’s abdomen or chest.
- Rates of postpartum hemorrhage have been on the rise around the world.
- Prior definitions of PPH included anyone who suffered a blood loss of 500 mL or more after a vaginal birth. A loss of 500 mL is equivalent to a routine blood donation and may be considered physiologic blood loss for a healthy adult giving birth.
- The U.S. reVITALize definition for early PPH includes (1) blood loss of ≥1000 mL or (2) any amount of blood loss that is accompanied by signs/symptoms of low blood volume within 24 hours after giving birth.
- PPH causes more than a quarter of maternal deaths globally, and in the U.S., it is thought to cause about 1 in 10 pregnancy-related deaths. A large study in the U.S. found that the risk of death from PPH was five times higher for Black birthing people compared to White birthing people—highlighting the massive racial injustices in our health care system.
- Care providers can prepare for obstetric emergencies before they occur: The American Academy of Family Physicians (AAFP) designed a comprehensive, evidence-based course called Advanced Life Support in Obstetrics (ALSO™) that can help lower the risk of blood loss ≥1,000 mL. We strongly encourage care providers to take advantage of ALSO or BLSO trainings!
- A meta-analysis of randomized trials shows that there are both potential benefits and potential harms to active management and expectant management.
- Compared to expectant management, active management reduced the average amount of blood loss at birth (by around 80 mL); the risk of blood loss greater than 500 mL (5% versus 15%); the risk of blood loss greater than 1,000 mL (0.9% versus 2.4%); the risk of blood transfusion (1% versus 3%); and the risk of anemia after birth (4% versus 7%) in a group that included both people at higher risk for PPH and those at lower risk for PPH. These benefits are likely due to the uterotonic alone (not early cord clamping or controlled cord traction). There were also downsides to active management: it increased the need for pain medication because of afterpains (5% versus 2%) and increases the risk of returning to the hospital for excess bleeding later on (3% versus 1%).
- At this time, there is no evidence that active management reduces the risk of blood loss ≥1,000 mL when focusing only on people considered to be at low risk of PPH.
- The evidence we have from randomized trials comes from settings where the care providers are more familiar with active than expectant management, and where interventions, including the use of synthetic oxytocin to start and speed up labor, are extremely common. Observational studies have not shown an increase in blood loss among pregnant people at low risk for PPH who experience expectant management with a skilled provider after a physiologic labor and birth.
- Researchers have written about best practices for both active management and expectant management.
- Best practices for active management include:
- Use synthetic oxytocin (Pitocin®) as the uterotonic.
- Wait until pulsation stops to clamp and cut the cord. Giving Pitocin® after delayed cord clamping has not been found to increase the risk of PPH. There is currently no evidence showing that the timing of routinely giving Pitocin® in the third stage of labor influences the rate of PPH.
- Consider giving Pitocin® either as an IV bolus pushed over one minute or as an IV infusion completed in around 30 minutes because recent studies show it may help to reduce blood loss better than IM injection.
- Controlled cord traction is optional for skilled providers; any benefits are minor, there is a potential for complications when performed incorrectly, and omitting it has not been shown to increase the risk of severe blood loss.
- Uterine massage is not recommended, but postpartum assessment of fundal tone is recommended to identify uterine atony.
- Best practices for expectant management can be better called “psychophysiological care” (Hastie and Fahy, 2009) and include:
- Await signs that the placenta has separated and monitor the birthing person for excessive blood loss (assess the volume of blood loss, the speed of blood flow, and the mother’s condition).
- Wait until pulsation stops to clamp and cut the cord.
- Support the birthing person in upright positions as they birth their placenta with pushing efforts or gravity.
- Encourage immediate skin-to-skin contact with the newborn and early opportunities to breastfeed/chestfeed.
- Foster a birth space that feels warm, calm, private, and safe to encourage the mother’s own oxytocin release.
- Uterine massage is not recommended (there is no evidence on using uterine massage without a uterotonic), but postpartum assessment of fundal tone is recommended to identify uterine atony.
- Have a uterotonic drug available to use as treatment if excess bleeding occurs.
- Best practices for active management include:
Bottom Line
Both modern active management (i.e. mixed management) and expectant management (especially “psychophysiological care”) during the third stage of labor are reasonable choices. The risks and benefits of each approach should be discussed with birthing families so they can make an informed choice about their care for the third stage. Informed consent discussions should include how individual risk factors, if present, could increase risk of PPH, how unplanned interventions and complications could increase risk of PPH (and mean reevaluating active versus expectant management at that point), and the birthing person’s values and preferences.
Early cord clamping is no longer recommended, and parents should be supported in their choice if they ask for the cord to remain unclamped until it has turned white and stopped pulsating.
Discussions can also include what the birth setting’s ability is to provide each type of third stage care. We list some of the best practices for each approach above. You can take this information and have a discussion with your care provider about how they usually handle the third stage of labor and how the birth setting could best support your desired approach.


References:
- Abedi, P., Jahanfar, S., Namvar, F., et al. (2016). Breastfeeding or nipple stimulation for reducing postpartum haemorrhage in the third stage of labour. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD010845. Click here. Free full text!
- Adnan, N., Conlan-Trant, R., McCormick, C., et al. (2018). Intramuscular versus intravenous oxy- tocin to prevent postpartum haemorrhage at vaginal delivery: randomised controlled trial. BMJ, 4. 362. Click here. Free full text!
- Aflaifel, N. and Weeks, A. (2012). Push, pull, squeeze, clamp: 100 years of changes in the management of the third stage of labour as described by Ten Teachers. BMJ, 345, e8270. Click here.
- American College of Nurse-Midwives, Midwives Alliance of North America, National Association of Certified Professional Midwives (2012). Supporting healthy and normal physiologic child- birth: a consensus statement by the American College of Nurse-Midwives, Midwives Alliance of North America, and the National Association of Certified Professional Midwives. J Midwifery Womens Health, 57(5), 529-532. Click here.
- American College of Obstetricians and Gynecologists (2017, Reaffirmed 2019). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol, 130, e168. Click here.
- Anger, H., Durocher, J., Dabash, R., et al. (2019). How well do postpartum blood loss and common definitions of postpartum hemorrhage correlate with postpartum anemia and fall in hemoglobin? PLoS One, 14, e0221216. Click here. Free full text!
- Association of Ontario Midwives (2016). AOM PPH CPG Work Group. Postpartum Hemorrhage. Accessed online November 13, 2019.
- Begley, C. M., Guilliland, K., Dixon, L., et al. (2012). Irish and New Zealand midwives’ expertise in expectant management of the third stage of kabour: the ‘MEET’ study. Midwifery, 28(6), 733-739. Click here.
- Begley, C. M., Gyte, G. M. L., Devane, D., et al. (2019). Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews, Issue 2. Art. No.: CD007412. Click here.
- Berghella, V. (2007). Obstetric Evidence Based Guidelines. Informa UK Ltd. Click here.
- Berghella, V., Lockwood, C. J., and Barss, V. (2019). UpToDate® Management of the third stage of labor: Drug therapy to minimize hemorrhage.
- Buckley, S. J. (2020). Leaving Well Alone: A Natural Approach to the Third Stage of labour. Accessed online February 4, 2020.
- California Maternal Quality Care Collaborative (CMQQC), OB Hem Task Force (2015). OB Hem Active Management of the Third Stage Labor. Accessed online March 27, 2020.
- Chamberlain G. (2006). British maternal mortality in the 19th and early 20th centuries. Journal of the Royal Society of Medicine, 99(11), 559–563. Click here. Free full text!
- Charles, D., Anger, H., Dabash, R., et al. (2018). Intramuscular injection, intravenous infusion, and intravenous bolus of oxytocin in the third stage of labor for prevention of postpartum hemorrhage: a three-arm randomized control trial. BMC Pregnancy Childbirth, 19, 38. Click here. Free full text!
- Chen, M., Chang, Q., Duan, T., et al. (2013). Uterine massage to reduce blood loss after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 122(2 Pt 1), 290–295. Click here.
- Cheyney, M., Bovbjerg, M., Everson, C., et al. (2014). “Outcomes of care for 16,924 planned home births in the United States: the Midwives Alliance of North America Statistics Project, 2004 to 2009.” J Midwifery Womens Health, 59(1), 17-27. Click here.
- Combs, C. A. and Laros, R. K. Jr. (1991). Prolonged third stage of labor: morbidity and risk factors. Obstetrics and Gynecology, 77(6), 863-867.
- Dashtinejad, E., Abedi, P. and Afshari P. (2018). Comparison of the effect of breast pump stimulation and oxytocin administration on the length of the third stage of labor, postpartum hemorrhage, and anemia: a randomized controlled trial. BMC Pregnancy Childbirth, 18(1), 293. Click here. Free full text!
- Declercq, E. R., Sakala, C., Corry, M. P., et al. (2013). Listening to MothersSM III: Pregnancy and Birth. New York: Childbirth Connection, May 2013. Click here. Free full text!
- Dencker, A., Smith, V., McCann, C., et al. (2017). Midwife-led maternity care in Ireland – a retrospective cohort study. BMC Pregnancy Childbirth, 17(1), 101. Click here. Free full text!
- Diaz, V., Abalos, E. and Carroli, G. (2018). Methods for blood loss estimation after vaginal birth.
- Cochrane Database of Systematic Reviews, Issue 9. Art. No.: CD010980. Click here. Free full text!
- Dixon, L., Tracy, S. K., Guililand, K. (2013). Outcomes of physiological and active third stage labour care amongst women in New Zealand. Midwifery, 29(1), 67-74. Click here.
- du Vigneaud, V., Ressler, C. and Trippett, S. (1953). The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem., 205(2), 949–57.
- Durocher, J., Dzuba, I. G., Carroli, G., et al. (2019). Does route matter? Impact of route of oxytocin administration on postpartum bleeding: A double-blind, randomized controlled trial. PLoS ONE 14(10): e0222981. Click here. Free full text!
- Erickson, E. N., Lee, C. S., and Emeis, C. L. (2017). Role of Prophylactic Oxytocin in the Third Stage of Labor: Physiologic Versus Pharmacologically Influenced Labor and Birth. J Midwifery Womens Health, 62(4), 418-424. Click here.
- Evensen, A., Anderson, J. M. and Fontaine, P. (2017). Postpartum Hemorrhage: Prevention and Treatment. Am Fam Physician. 95(7), 442-449. Click here. Free full text!
- Fahy, K., Hastie, C., Bisits, A., et al. (2010). Holistic physiological care compared with active management of the third stage of labour for women at low risk of postpartum hemorrhage: a cohort study. Womens Birth, 23(4), 146-52. Click here.
- Farrar, D., Tuffnell, D., Airey, R., et al. (2010). Care during the third stage of labour: a postal survey of UK midwives and obstetricians. BMC Pregnancy Childbirth 10 (1), 23. Click here. Free full text!
- Ford, J. B., Roberts, C. L., Simpson, J. M., et al. (2007). Increased postpartum hemorrhage rates in Australia. International Journal of Gynecology & Obstetrics, 98(3), 237–243. Click here.
- Fuchs, A. R. and Fuchs, F. (1984). Endocrinology of human parturition: a review. Br J Obstet Gynaecol, 91(10), 948–967. Click here.
- Funai, E. F., Norwitz, E. R., Lockwood, C. J., et al. (2019). UpToDate® Management of normal labor and delivery.
- Grobman, W., Lockwood, C. J. and Barss, V. A. (2019). UpToDate® Induction of labor with oxytocin.
- Gyamfi-Bannerman, C., Srinivas, S. K., Wright, J. D., et al. (2018). Postpartum hemorrhage outcomes and race. Am J Obstet Gynecol. 219(2), 185.e1–185.e10. Click here.
- Hancock, A. Weeks, A. D. and Lavender, D. T. (2015). Is accurate and reliable blood loss estimation the ‘crucial step’ in early detection of postpartum haemorrhage: an integrative review of the literature. BMC Pregnancy Childbirth, 15, 230. Click here. Free full text!
- Hastie, C. and Fahy, K. M. (2009). Optimising psychophysiology in third stage of labour: theory applied to practice. Womens Birth, 22(3), 89-96. Click here.
- Hofmeyr, G. J., Abdel-Aleem, H. and Abdel-Aleem, M. A. (2013). Uterine massage for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews, Issue 7. Art. No.: CD006431. Click here.
- Hofmeyr, G. J., Mshweshwe, N. T. and Gülmezoglu, A. M. (2015). Controlled cord traction for the third stage of labour. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD008020. Click here. Free full text!
- Homer, C. S., Leap, N., Edwards, N., et al. (2017). Midwifery continuity of carer in an area of high socio-economic disadvantage in London: A retrospective analysis of Albany Midwifery Practice outcomes using routine data (1997-2009). Midwifery, 48, 1-10. Click here. Free full text!
- Hutton, E. K. and Hassan, E. S. (2007). Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 297(11), 1241‐1252. Click here.
- International Confederation of Midwives (2008, Revised 2017). Role of the Midwife in Physiological Third Stage Labour: ICM Position Statement. Adopted at Glasgow Council meeting, 2008 and revised at Toronto Council meeting, 2017. Due for next review 2023. Click here. Free full text!
- Jangsten, E., Bergh, I., Mattson, L. A., et al. (2011). Afterpains: a comparison between active and expectant management of the third stage of labor. Birth, 38(4), 294-301. Click here.
- Kavanagh, J., Kelly, A. J. and Thomas, J. (205). Breast stimulation for cervical ripening and induction of labour. Cochrane Database Syst Rev. (3), CD003392. Click here.
- Kjerulff, K. H., Attanasio, L. B., Edmonds, J. K., et al. (2017). Labor induction and cesarean delivery: A prospective cohort study of first births in Pennsylvania, USA. Birth (Berkeley, Calif.), 44(3), 252–261. Click here. Free full text!
- Kramer, M. S., Berg, C., Abenhaim, H., et al. (2013). Incidence, risk factors, and temporal
- trends in severe postpartum hemorrhage. Am J Obstet Gynecol, 209(5), 449.e1-7. Click here.
- Kramer, M. S., Dahhou, M., Vallerand, D., et al. (2011). Risk factors for postpartum hemorrhage: can we explain the recent temporal increase? J Obstet Gynaecol Can., 33(8):810-819. Click here.
- Main, E. K., Cape, V., Abreo, A., et al. (2017). Reduction of severe maternal morbidity from hemorrhage using a state perinatal quality collaborative. Am J Obstet Gynecol. 216(3), 298.e1‐298.e11. Click here.
- Magon, N. and Kalra, S. (2011). The orgasmic history of oxytocin: Love, lust, and labor. Indian journal of endocrinology and metabolism, 15 Suppl 3(Suppl3), S156–S161. Click here. Free full text!
- Martin, J. A., Hamilton, B. E., Osterman, M. J. K., et al. (2019). Births: Final data for 2018. National Vital Statistics Reports; vol 68, no 13. Hyattsville, MD: National Center for Health Statistics. Click here. Free full text!
- Mavrides, E., Allard, S., Chandraharan, E., et al. on behalf of the Royal College of Obstetricians and Gynaecologists (2016). Prevention and management of postpartum haemorrhage. BJOG, 124, e106–e149. Click here. Free full text!
- McDonald, S. J., Middleton, P., Dowswell, T., et al. (2013). Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database of Systematic Reviews, Issue 7. Art. No.: CD004074. Click here. Free full text!
- Meisenberg, G. and Simmons, W. H. (1983). Peptides and the blood-brain barrier. Life Sciences, 32(23), 2611–2623. Click here.
- National Institute for Health and Care Excellence (2019). Care in third stage of labour. Accessed online November, 13, 2019. Available at: https://pathways.nice.org.uk/pathways/intrapartum-care/care-in-third-stage-of-labour
- Oberg, A. S., Hernandéz-Diaź, S., Frisell, T., et al. (2014). Genetic contribution to postpartum haemorrhage in Swedish population: cohort study of 466,686 births. BMJ, 349:g4984. Click here. Free full text!
- Oguz Orhan, E., Dilbaz, B., Aksakal, S. E., et al. (2014). Prospective randomized trial of oxytocin administration for active management of the third stage of labor. International Journal of Gynecology & Obstetrics, 127(2), 175–179. Click here.
- Oladapo, O. T., Okusanya, B. O., Abalos, E. (2018). Intramuscular versus intravenous prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews, Issue 9. Art. No.: CD009332. Click here. Free full text!
- OpenStax, Anatomy & Physiology (2016). OpenStax CNX. Maternal Changes During Pregnancy, Labor, And Birth. Accessed online March 27, 2020.
- Quintana, D. S., Smerud, K. T, Andreassen, O. A., et al. (2018). Evidence for intranasal oxytocin delivery to the brain: recent advances and future perspectives. Therapeutic Delivery, Vol. 9, No. 7. Click here. Free full text!
- Razvi, K., Chua, S., Arulkumaran, S., et al. (1996). A Comparison Between Visual Estimation and Laboratory Detennination of Blood Loss During the Third Stage of Labour. Aust N Z J Obstet Gynaecol, 36, 152–4. Click here.
- Reed, R., Gabriel, L. and Kearney, L. (2019). Birthing the placenta: women’s decisions and experiences. BMC Pregnancy Childbirth. 19(1), 140. Click here. Free full text!
- Salati, J. A., Leathersich, S. J., Williams, M. J., et al. (2019). Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews, Issue 4. Art. No.: CD001808. Click here.
- Schorn, M.N., Dietrich, M.S., Donaghey, B., et al. (2017). US Physician and midwife adherence to active management of the third stage of labor international recommendations. J. Midwifery Women’s Health 62 (1), 58–67. Click here.
- Sheiner, E., Sarid, L., Levy, A., et al. (2005). Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. Journal of Maternal-Fetal & Neonatal Medicine, 18(3), 149–54. Click here.
- Society of Obstetricians and Gynecologists of Canada (2018). No. 235-Active Management of the Third Stage of Labour: Prevention and Treatment of Postpartum Hemorrhage. J Obstet Gynaecol Can, 40(12), e841-e855. Click here.
- Soltani, H., Hutchon, D. R. and Poulose, T. A. (2010). Timing of prophylactic uterotonics for the third stage of labour after vaginal birth. Cochrane Database of Systematic Reviews, Issue 8. Art. No.: CD006173. Click here.
- Sorensen, B., Rasch, V., Massawe, S., et al. (2011). Advanced Life Support in Obstetrics (ALSO) and post-partum hemorrhage: a prospective intervention study in Tanzania. Acta Obstet Gynecol Scand. 90, 609-614. Click here.
- Takahata, K., Horiuchi, S., Tadokoro, Y., et al. (2018). Effects of breast stimulation for spontaneous onset of labor on salivary oxytocin levels in low-risk pregnant women: A feasibility study. PLoS One, 13(2), e0192757. Click here. Free full text!
- Uvnäs-Moberg, K., Ekström-Bergström, A., Berg, M., et al. (2019). Maternal plasma levels of oxytocin during physiological childbirth – a systematic review with implications for uterine contractions and central actions of oxytocin. BMC pregnancy and childbirth, 19(1), 285. Click here. Free full text!
- van Stralen, G., von Schmidt Auf Altenstadt, J. F., Bloemenkamp, K W., et al. (2016). Increasing incidence of postpartum hemorrhage: the Dutch piece of the puzzle. Acta Obstet Gynecol Scand., 95(10), 1104-10. Click here.
- Vogel, J. P., Williams, M., Gallos, I., et al. (2019). WHO recommendations on uterotonics for postpartum haemorrhage prevention: what works, and which one?. BMJ Glob Health. 4(2), e001466. Click here. Free full text!
- Weeks, A. D. (2008). The retained placenta. Best Practice & Research Clinical Obstetrics & Gynaecology, 22(6), 1103–1117. Click here.
- Weeks, A., Berghella, V. and Barss, V. A. (2019). UpToDate® Retained placenta after vaginal birth.
- World Health Organization (2012). WHO recommendations for the prevention and treatment of postpartum haemorrhage. Accessed online February 6, 2020.
- World Health Organization (2018). Uterotonics for the prevention of postpartum haemorrhage. Accessed online October 30, 2019.
- World Health Organization (2019). Optimal timing of cord clamping for the prevention of iron deficiency anaemia in infants. Accessed online November 13, 2019.
Resources:
- Advanced Life Support in Obstetrics (ALSO) training was designed by the American Academy of Family Physicians (AAFP) to prepare maternity care providers to effectively manage potential emergencies during pregnancy and childbirth, including PPH. You can find information about how to hold a course, attend a course, or instruct a course on the AAFP website here!
- The California Maternal Quality Care Collaborative (CMQCC) developed the OB Hemorrhage Toolkit V 2.0 in 2015 (CMQCC, OB Hem Task Force, 2015). You can download the toolkit here!
- Sarah Buckley’s 2015 report on the Hormonal Physiology of Childbearing: Evidence and Implications for Women, Babies, and Maternity Care is available at: ChildbirthConnection.org/HormonalPhysiology.
- Cochrane authors independently assess the quality of the evidence in their reviews using the GRADE approach. We indicated whether the evidence was considered high, moderate, low, or very low quality for many of the outcomes discussed in this article. You can find all of the details on the GRADE approach to assessing the quality of evidence in the GRADE Handbook here.
Acknowledgements
We are grateful to our reviewers for this article— Margaret Buxton, CNM, DNP, Director of Clinical Operations, Baby & Co; Audrey Lyndon, PhD, RNC, FAAN, Professor of Nursing and Medicine, Assistant Dean for Clinical Research at NYU Rory Meyers College of Nursing; Debbie Newton, APRN, CNM, MSN; Paula Richards, MSN, RNC-OB, C-EFM; Nathan Riley, Obstetrician, Palliative Medicine; and Shannon J. Voogt, MD, Board-Certified in Family Medicine.
We also wish to thank Cristen Pascucci for her medical editing assistance.
Cover Photo Credit: Katie McKinney Photography
Birth Professionals:
Join others who also want to help bring evidence-based care to their local community.
Also, gain complimentary access to a printable library of our Signature Articles, 20+ hours of CE courses, a private community, and more.
Buy EBB Inspirational T-shirts, Due Date Buttons & Birth Affirmation Cards




Stay empowered, read more :
EBB 310 – Doulas & Nurses Advocating Together for Positive Shifts in Birth Culture with Joyce Dykema, EBB Instructor & Brianna Fields, RN
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | YouTube Dr. Rebecca Dekker is joined by Joyce Dykema, doula and EBB Instructor, and Brianna Fields, a labor and delivery nurse, to discuss advocating for positive shifts in...
EBB 308 – The Intersection of Environmental Justice and Midwifery Care with Dr. Tanya Khemet Taiwo
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | YouTube Curious which toxins should be most avoided for people of reproductive age and their children? In this episode, Dr. Tanya Khemet Taiwo, LM, CPM, MPH, PhD unravels the...
EBB 307 – Unexplained Infertility, Endometriosis, and a Birth Center Birth Story with Ellora La Shier, EBB Childbirth Class Graduate
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | GoogleIn this episode, Ellora La Shier, graduate of the EBB Childbirth Class, shares about her struggle with six years of unexplained infertility and how it impacted her...



