
Rebecca Dekker
PhD, RN
Evidence on: Induction for Gestational Diabetes
Originally published on July 3, 2012 and updated on April 3, 2019, All Rights Reserved. Please read our Disclaimer and Terms of Use. For a printer-friendly PDF, become a Professional Member to access our complete library.
Gestational diabetes mellitus (GDM) is defined as high blood glucose (high blood sugar) that develops during pregnancy (ADA, 2018). We cover the evidence on diagnosing GDM in a separate Evidence Based Birth® article here. Two of the main questions that come up in caring for pregnant people with GDM are the following: Should labor be induced? And, if induction for gestational diabetes is chosen, when should it occur?
The main alternative to labor induction for gestational diabetes is expectant management. Choosing expectant management means you decline elective induction for gestational diabetes now, and instead plan to wait for labor to start on its own. With expectant management, you could also be induced later if complications develop, or you could be induced electively further along in the pregnancy.
What is gestational diabetes?
We encourage you to read the beginning of the Evidence Based Birth® Signature Article on Diagnosing GDM. There, we define several important terms and explain what’s going on in the body when someone has GDM.
Gestational diabetes is a complex topic, so we reached out to experts in the field for help describing the condition. The background information in the Diagnosing GDM article will give you a basic understanding before moving on to the evidence on induction for gestational diabetes. Pre-existing diabetes (Type 1 and Type 2) are managed differently from GDM and are not covered in this article.
What problems can result from gestational diabetes?
In the Evidence Based Birth® Signature Article on Diagnosing GDM, we discuss the “Hyperglycemia and Adverse Pregnancy Outcomes” (HAPO) study in detail. This study is the most important research that has ever been done on the link between maternal blood sugar and risk of poor birth outcomes (HAPO, 2008). The key finding from the HAPO study was that the relationship between a mother’s blood sugar levels and the risk of poor birth outcomes is continuous. This means that there is no specific cutoff for risk—the risk of poor outcomes increases step-by-step with every small increase in blood sugar levels, even at levels not considered to be GDM.
The HAPO study and other studies have linked GDM to higher rates of:
(HAPO, 2008; England et al. 2009; Tobias et al. 2017; Clausen et al. 2009)
- Pre-eclampsia
- Fetal high blood sugar
- First-time Cesarean
- Premature birth
- Higher birth weight/having a large baby
- Shoulder dystocia or birth injury
- Newborn intensive care
- Newborn jaundice
- Newborn low blood sugar
- The mother developing diabetes and/or heart disease later in life
- The baby developing excess body weight and/or diabetes later in life
How common is induction for gestational diabetes?
There is very little data on how often people with GDM are induced because of their diagnosis. In the U.S., birth certificates do not accurately track labor induction. Birth certificates also do not provide accurate info about the reasons for induction (Declercq et al. 2013; Dublin et al. 2014).
We found one retrospective study that looked at more than 330,000 births in the U.S. from 2001 to 2007 and described trends in labor induction (Dublin et al. 2014). The people in the study came from six health insurance plans, many different hospitals and regions, and represented a large and diverse population. Health insurance plan data was linked to birth certificate data in order to improve accuracy compared to using birth certificate data alone. The researchers stated that induction occurred if it was documented either in the health insurance plan records or birth certificate data. One limitation of this study is that all of the participants were insured, and less than 6% were enrolled in Medicaid, so the findings may differ for those without private insurance.
Overall, 30% of labors were induced. When they looked at reasons for induction, 59% of the labors were induced for an accepted medical reason and 41% were considered to be elective inductions. There is no official definition of elective induction, so the researchers defined an elective induction as an induction that occurred before 40 completed weeks of pregnancy without one of the listed medical indications. They did not consider a suspected big baby to be a valid medical indication.
The researchers found that diabetes (gestational diabetes or pre-existing diabetes) was the medical reason given for 10% of the medically indicated inductions. Unfortunately, the authors did not distinguish between GDM and diabetes that was present before the pregnancy, so we don’t know how many of those were specifically induction for gestational diabetes.
Does gestational diabetes always mean induction of labor?
Since people with GDM and their babies are at increased risk of pregnancy complications, some care providers encourage women with GDM to plan an early birth (usually elective induction) at or near term instead of waiting for labor to start on its own. However, it’s important that we have evidence to show that planned early birth actually benefits mothers with GDM and their babies before recommending medical induction for gestational diabetes as routine.
Evidence from randomized controlled trials
Biesty et al. (2018) published a Cochrane review in which they searched for randomized controlled trials that compared planned early birth (elective induction or Cesarean) at or near term (37 to 40 weeks’ gestation) versus expectant management for people with GDM. Unfortunately, they found only one randomized controlled trial to include in the review (Alberico et al. 2017).
Side note: In an earlier version of this Evidence Based Birth® article, we cited another trial on this topic (Kjos et al. 1993), but that study was not included in the Cochrane review because it included women with pre-existing Type 2 diabetes as well as those with GDM.
The GINEXMAL Trial
The large Alberico et al. (2017) trial (called the GINEXMAL Trial) took place at eight hospitals across Italy, Slovenia, and Israel. The participants all had GDM as diagnosed by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria and no other maternal or fetal medical problems. The IADPSG diagnostic criteria are described in our Signature Article on Diagnosing GDM. In the GINEXMAL trial, 214 participants were randomly assigned to an induction of labor between 38 weeks, 0 days and 39 weeks, 0 days of pregnancy (i.e., early term induction). The other 211 participants were assigned to wait for labor to start on its own until 41 weeks, 0 days, as long as no medical problems developed (i.e., expectant management). They received fetal monitoring tests twice per week until birth.
When they compared the groups at baseline (right after randomization), fewer people in the early term induction group used medication to manage their GDM, compared with those in the expectant group (56% used medication in the early term induction group versus 76% in the expectant management group). This means that the groups weren’t completely similar to begin with. However, the number of people with well-controlled blood sugar levels was the same between the groups, so the authors think it probably didn’t affect the study results (Alberico et al. 2017).
The researchers found that for babies, there were no differences between groups in the number of large babies (more than 8 pounds, 13 ounces, or 4,000 grams), or the risk of shoulder dystocia, breathing problems, low blood sugar, or intensive care. More babies in the early term induction group experienced jaundice (10% versus 4%).
For mothers, there was no difference between groups in the risk of Cesarean, birth with forceps/vacuum, postpartum hemorrhage, intensive care, or intact perineum. No deaths occurred among mothers or babies in the study.
The quality of the evidence from this study was considered low to very low because of high risk of bias (women and their care providers were not blinded). Also, the study was too small to look at differences in rare outcomes, such as death (Biesty et al. 2018). There was a small amount of crossover between groups, when people didn’t stick with their random group assignments: 11% of people assigned to early term induction were not induced as intended and 9% of people assigned to expectant management actually received an early elective induction. Regardless, the researchers concluded that the amount of crossover was not significant enough to change the results (Alberico et al. 2017).
Not surprisingly, early term induction was linked to overall lower birth weights for newborns. However, the decrease in birth weights with early term induction did not make a difference for any of the clinically important outcomes, including the number of babies more than 8 pounds, 13 ounces (4,000 grams), Cesareans, or shoulder dystocia. In the study, shoulder dystocia occurred in three births in the early induction group and one birth in the expectant management group and this difference was not significant. All four cases of shoulder dystocia were resolved without any problems.
In theory, a reduction in birth weights could reduce the risk of shoulder dystocia, but since shoulder dystocia was so infrequent, a larger randomized trial would be needed to study this outcome. Also, it would likely take a very large number of women with GDM to ‘treat’ with early term induction in order to prevent one event of shoulder dystocia. In the same way, it would take a very large number of women with GDM to determine if early term induction would prevent one event of stillbirth, as we will discuss further on.
Evidence from observational studies
Given that there is only one randomized, controlled trial on this topic, it is important to look at observational studies on this topic. In observational studies, there is no “assignment” to an induction or expectant management—instead, researchers usually look back in time at what happened when women and their care providers decided to induce or use a policy of expectant management.
We searched PubMed for non-randomized studies that compared early induction for gestational diabetes at or near term versus expectant management. We restricted the search to studies published since 2010, since earlier studies usually included people with pre-existing Type 2 diabetes in addition to those with GDM (Witkop et al. 2009).
Four studies looked specifically at birth outcomes after early induction for gestational diabetes versus expectant management. Table 1 provides details about these four observational studies. We will also summarize their results below, starting with what the studies found about outcomes for mothers, and then what they found for babies.
Table 1: Observational studies of early induction vs. expectant management for people with GDM
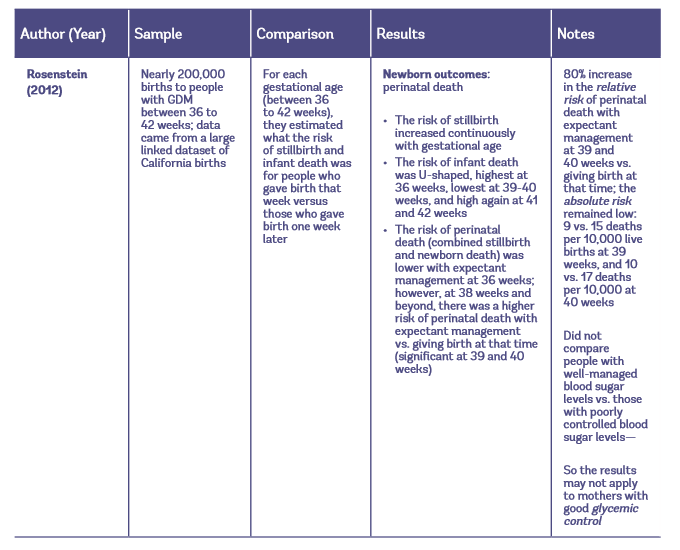
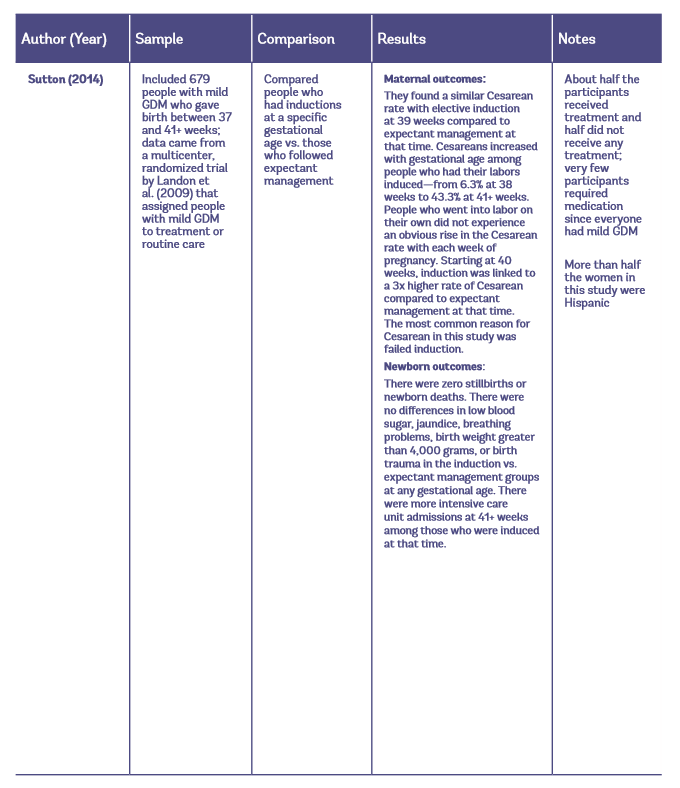
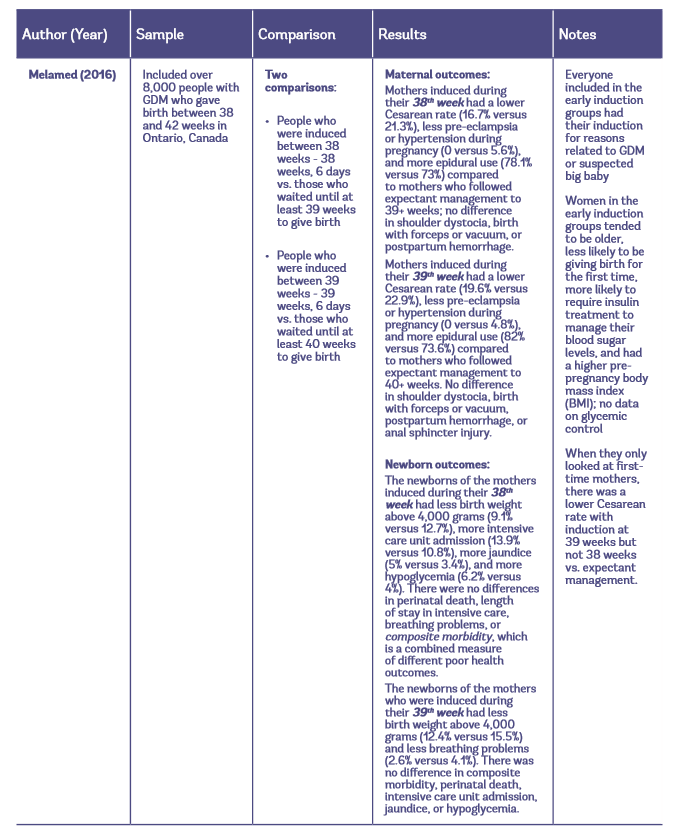
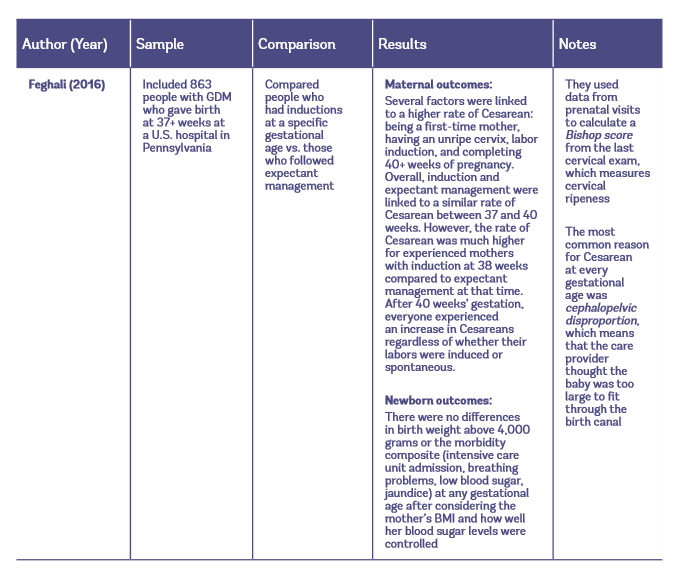
Maternal Outcomes with Elective Induction vs. Expectant Management
The largest study to look at maternal outcomes included over 8,000 pregnant people with GDM. They found that inducing labor at 38 or 39 weeks for GDM is linked to a lower rate of Cesareans, less pre-eclampsia/hypertension, and more epidural use compared to expectant management at those times (Melamed et al. 2016). When they looked exclusively at first-time mothers, there was no benefit to inducing labor at 38 weeks; only 39-week induction was linked to a lower rate of Cesareans compared to following expectant management to 40+ weeks (19.6% versus 22.9%).
In another study, researchers also reported cervical ripeness and whether the mother had given birth before (Feghali et al. 2016). These researchers found that people with GDM who had had a previous vaginal birth significantly increased their risk of Cesarean by attempting induction before 39 weeks, especially with an unripe cervix. Therefore, based on this and the Melamed et al. (2016) study, it appears that 38-week elective induction for gestational diabetes should not be routinely recommended to first-time or experienced mothers. In the Feghali et al. (2016) study, induction at 39 weeks’ gestation resulted in a similar Cesarean rate compared to expectant management at that time. After 40 weeks’ gestation, everyone experienced an increase in Cesareans, regardless of whether their labors were induced or spontaneous.
Sutton et al. (2014) also found no difference in the Cesarean rate with elective induction at 39 weeks compared to expectant management at that time. And similarly, they found that the rate of Cesareans significantly increased with gestational age. However, contrary to the previous study by Feghali et al. (2016), this result was seen only among people who had their labors induced, not those who went into labor spontaneously.
To summarize these findings on the Cesarean rate, the largest study found a lower Cesarean rate with 39-week induction and the other two found no difference between 39-week induction and expectant management at that time. Overall, the Cesarean rate among people with GDM appears to increase with gestational age after 40+ weeks with both induction and spontaneous labor.
Why might Cesareans go up after 40 weeks?
Perhaps continuing a pregnancy to 40+ weeks leaves more time for potential medical problems to develop. In other words—the pregnant person is more likely to become “high-risk.” It could also be that care providers are quicker to recommend Cesarean at later gestational ages (they may be less patient with the labor before labeling it as “failed.”)
Some researchers suggest that an increase in the baby’s weight could contribute to an increase in Cesarean at a later gestational age. However, it could also be the provider’s perception that the risk of having a big baby has gone up—leading to an increased risk of Cesarean, even if the baby is born a normal weight. (See our article on Big Babies for research on how the suspicion of a big baby—not big babies themselves—leads to higher Cesarean rates.) There is also limited evidence from animal studies that diabetes during pregnancy can harm the growth and function of the placenta, which could make it more difficult for a baby to cope with labor as gestational age increases (Vambergue and Fajardy, 2011).
Newborn Outcomes with Elective Induction vs. Expectant Management
The largest observational study on GDM and induction found that newborns of mothers who are induced during their 38th week of pregnancy tend to have more health problems than newborns of mothers who are induced during their 39th week of pregnancy. Compared to expectant management, 38-week induction is linked to fewer babies with birth weight above 4,000 grams (fewer large babies), but higher rates of intensive care unit admission, jaundice, and low blood sugar (Melamed et al. 2016).
On the other hand, compared to expectant management at that time, 39-week induction is linked to fewer cases of birth weight above 4,000 grams (fewer large babies) and fewer cases of breathing problems, without an increase in intensive care unit admission, jaundice, or low blood sugar. The risk of intensive care unit admissions goes up again after 41 weeks among people who have their labors induced at that time (Sutton et al. 2014).
Stillbirth
One study focused on the risk of stillbirth and infant death for people with GDM who give birth at different gestational ages (Rosenstein et al. 2012). This study is important, because many women have reached out to us at Evidence Based Birth® and stated that their care providers told them they should be induced for GDM because of the risk of stillbirth and infant death (when a fetus dies in utero or within one year of life), also called perinatal death. When care providers suggest that early induction reduces perinatal death among people with GDM they are probably referencing findings from the large retrospective study by Rosenstein et al. (2012), which looked back in time at birth outcomes among people with GDM. This study found that expectant management at 39 and 40 weeks carried an 80% higher relative risk of perinatal death compared to giving birth at that time.
Relative risk is a useful way of comparing risk in one group to another. However, we’re talking about very rare events, so the relative risk tends to cause people to overestimate the effect— in this case, overestimating the risks of continuing the pregnancy (Noordzij et al. 2017). The absolute risk of perinatal death, or the actual chance of the event occurring, is very low whether a woman with GDM chooses to be induced or follow expectant management.
At 39 weeks, the absolute risk of stillbirth or newborn death was 9 deaths per 10,000 for people who gave birth versus 15 deaths per 10,000 with expectant management for one more week. At 40 weeks, the absolute risk for those who gave birth was 10 deaths per 10,000 who gave birth versus 17 deaths per 10,000 for those who followed expectant management for one more week. The number needed to treat, or the number of women who would need to be treated with induction to prevent one death at 39 or 40 weeks’ gestation was very high—around 1,500 and 1,300 women with GDM, respectively.
Other Research on Induction for Gestational Diabetes
We wanted to briefly mention two other studies that have been published since 2010. These studies also looked at induction for gestational diabetes, but not according to whether people chose early induction versus expectant management.
A decision analysis that created a theoretical (made-up) group of 100,000 women with diet-controlled GDM found that inducing everyone at 38 weeks or 39 weeks of gestation would reduce overall perinatal death without increasing Cesarean rates (Niu et al. 2014). This computer-based study used risk estimates from the literature, but they didn’t report details of how they did their literature review to find these estimates. They concluded that giving birth at 38 weeks would prevent 48 stillbirths but lead to 12 more infant deaths and 21 more cases of cerebral palsy compared to giving birth at 39 weeks. Also, according to their model, giving birth at 38 weeks would slightly reduce the rate of maternal deaths from 16.2 per 100,000 to 15.4 per 100,000.
A retrospective study from Israel compared 240 people with GDM who were induced between 37 and 40 weeks to 454 people who were induced for term PROM (when the mother’s water breaks before the start of regular labor contractions) (Bas-lando et al. 2014). This is not an appropriate choice for a comparison group, but the authors wanted to see if elective induction for gestational diabetes is linked to a higher Cesarean rate compared to other reasons for elective induction. They found that elective induction with GDM is indeed linked to a higher Cesarean rate compared to elective induction with term PROM (17% versus 11%). Although this may be true, we would caution that these two groups do not make for a reliable comparison because people with term PROM tend to be easier to induce—the rupture of membranes can induce labor in itself and also can mean that the body was ready to go into labor.
Are there effective treatments for gestational diabetes that reduce the risk of poor outcomes?
If you have GDM, treatment with diet changes, exercise, and sometimes medicine, is necessary to maintain healthy blood sugar levels. With effective treatment, it is possible for someone with GDM to reduce the risk of having complications from the condition, such as a big baby. Importantly, when the risk of complications from GDM is reduced with treatment, then there is less potential benefit from labor induction for gestational diabetes.
Overview of Cochrane systematic reviews on treatments for gestational diabetes
Several Cochrane reviews have looked at different treatments to improve pregnancy outcomes for people with GDM. Recently, Cochrane researchers published an overview of 14 of these systematic reviews, and pooled data from 10 of the reviews in a giant meta-analysis (128 trials, nearly 18,000 mothers) (Martis et al. 2018). They looked at dietary interventions, exercise programs, insulin and oral-glucose-lowering drugs, supplements, combination lifestyle interventions, and obstetric management strategies (induction or planned Cesarean).
The only intervention found to provide effective treatment for GDM leading to health benefits for mothers and babies was lifestyle changes that combined two or more interventions. This data came from the Cochrane review and meta-analysis by Brown et al. (2017a). At a minimum, the lifestyle intervention included healthy eating, exercise, and self-monitoring of blood sugar levels. Of the people assigned to lifestyle intervention, 10% also received pharmacological (drug) interventions, such as insulin or oral anti-diabetic therapies if they needed additional help managing their blood sugar levels.
People randomly assigned to lifestyle intervention versus usual care were 40% less likely to have babies large for gestational age, defined as weighing more than 90% of other babies (6 trials, 2,994 participants). They were also 62% less likely to experience shoulder dystocia (5 trials, 2,894 babies). It’s also important to note that the majority of participants (90%) achieved these benefits from lifestyle changes alone (without any medication).
There was no clear evidence of a difference between groups for the risk of pre-eclampsia, Cesarean, the mother developing Type 2 diabetes, perineal trauma, or induction of labor. While the difference in the rate of induction between groups was not statistically significant, the authors mention several times that there was a trend toward lifestyle intervention increasing the risk of induction of labor. They found this trend worrisome enough to conclude “lifestyle intervention may increase the number of inductions, causing possible harm.” However, this trend was not statistically significant, and all it means is that we need more research on whether prescribing lifestyle intervention can lead to an increase in the risk of induction.
Seven of the 14 meta-analyses reported on stillbirth and infant death. There was no evidence of a difference between groups for any of the treatment interventions. So we do not have evidence from meta-analyses of randomized controlled trials (the highest level of scientific evidence) that treating someone with GDM lowers their risk of experiencing stillbirth or infant death.
Systematic review and meta-analysis by Farrar et al. (2017)
This other recent study also looked at whether treatment for GDM improves the health of the mother and baby. It included 12 trials that compared ‘bundles of care’ (starting with lifestyle changes then using medication as necessary to lower blood sugar levels) versus routine care. They found that bundles of care cut the risk of large birth weight by 50% and shoulder dystocia by 60%. They did not find a significant difference in the risk of pre-eclampsia, Cesarean, newborn intensive care, newborn low blood sugar, preterm birth, low Apgar scores, use of forceps/vacuum, or labor induction. In two of the trials, mothers reported higher quality of life after treatment for GDM.
Treatment for gestational diabetes improves outcomes
The good news is that treatment for gestational diabetes improves outcomes. If you are diagnosed with GDM and you choose treatment (starting with advice about diet/exercise and self-monitoring of blood sugar levels, followed by medication as needed), you are less likely to have a baby that weighs more than 8 pounds, 13 ounces or is large for gestational age. You are also less likely to experience shoulder dystocia.
There are still a great many things we don’t know about treatments for GDM. There is very little research on potential long-term health benefits from treatment for mothers or babies. As far as safety, insulin does not cross the placenta, and thus is highly unlikely to cause any harm to the baby (Martis et al. 2018). On the other hand, oral antidiabetic medications (i.e. metformin and glyburide) do cross the placenta and there is limited information about the long-term effects of these medications on the baby. We don’t know which combination lifestyle intervention is most effective, or which medications (insulin or oral anti-diabetic medications) work best for individuals. For example, insulin is considered the preferred medication for women in pregnancy, but new evidence suggests that insulin may increase the risk of hypertensive disorders of pregnancy compared to oral medications for some individuals (ACOG, 2018; Martis et al. 2018).
We also don’t know much about the psychological impact of treatment on pregnant people. Being diagnosed and treated for GDM may cause stress for some women. However, in two of the trials in the Farrar et al. (2017) meta-analysis, mothers reported higher quality of life after treatment for GDM, including lower levels of postpartum depression. Mothers diagnosed with GDM face more medical appointments (to meet with a registered dietitian, a diabetes educator, or both) and they are told to carefully watch what they eat and monitor blood sugar levels several times a day (NIH, 2013). Testing supplies, blood sugar medication (if needed), and extra monitoring all come with significant costs, which are not always fully covered by insurance.
Mothers who require medication to manage their blood sugar levels also face additional fetal testing (monitoring the baby and levels of amniotic fluid). There is no consensus on whether people with diet-controlled GDM should receive routine fetal testing. The specific type of testing and how often to test varies from provider to provider (ACOG, 2018).
Recently updated practice guidelines on labor induction with gestational diabetes
The American College of Obstetricians and Gynecologists (ACOG) advises against inducing labor before 39 weeks in people with GDM who have well-controlled blood sugar levels with diet and exercise alone. For these women, they recommend that expectant management is appropriate up to 40 weeks, 6 days. For people with GDM who have well-controlled blood sugar levels with medication, ACOG recommends birth between 39 weeks, 0 days to 39 weeks, 6 days. ACOG guidance suggests even earlier inductions for people with poorly controlled blood sugar levels, but it’s important to consider the tradeoffs, since prematurity also carries risks (ACOG, 2018).
In the United Kingdom, guidelines also advise people with GDM to give birth no later than 40 weeks, 6 days (NICE, 2015).
The Polish Gynecological Society recommends that people with GDM consider induction after 39 weeks (Bomba-Opoń et al. 2017).
In Canada, the current recommendation is that pregnant people with GDM should be offered an induction between 38 to 40 weeks, depending on their blood sugar control and other risk factors (Berger et al. 2016).
Conclusion
- At this time, there is no evidence from randomized controlled trials to support routinely inducing labor at 38 or 39 weeks for everyone with GDM. The one randomized trial on induction for gestational diabetes failed to find any benefits for the mother or baby from elective induction between 38 weeks, 0 days and 39 weeks, 0 days of pregnancy versus waiting for labor to start on its own until 41 weeks, 0 days, as long as no medical problems developed. Importantly, this trial was not large enough to detect a difference in stillbirth.
- There is some evidence from observational studies that people with GDM who give birth at 39 or 40 weeks have a lower relative risk of perinatal death compared to those who continue the pregnancy beyond 40 weeks. However, the absolute risk of perinatal death is low whether a mother chooses planned early birth or waits for labor to start on its own. Since the evidence on perinatal death comes from a large study that used administrative data, the researchers could not comment on the glucose control of the women, so it’s not clear if the results apply to mothers with well-managed blood sugar levels.
- At 39 weeks, the absolute risk of stillbirth or newborn death was 9 deaths per 10,000 for people who gave birth versus 15 deaths per 10,000 with expectant management for one more week.
- At 40 weeks, the absolute risk of stillbirth or newborn death was 10 deaths per 10,000 for people who gave birth versus 17 deaths per 10,000 with expectant management for one more week.
- The largest observational study to look at maternal and newborn outcomes found that inducing labor at 39 weeks is linked to a lower rate of Cesarean and fewer cases of pre-eclampsia/hypertension compared to waiting until at least 40 weeks to give birth. Newborns of mothers who are induced during their 39th week of pregnancy are less likely to weigh more than 4,000 grams and less likely to have breathing problems compared to those born at 40+ weeks. It is possible that these potential benefits of early induction do not apply to mothers with GDM who have well-managed blood sugars.


References:
- Alberico, S., Erenbourg, A., Hod, M., et al. (2017). Immediate delivery or expectant management in gestational diabetes at term: the ginexmal randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology;124(4):669–77.
- American College of Obstetricians and Gynecologists (2016). Fetal macrosomia. Practice Bulletin No. 173. Obstet Gynecol;128:e195–209.
- American College of Obstetricians and Gynecologists (2018). “ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus.” Obstetrics and Gynecology 131(2): e49-e64.
- American Diabetes Association (2018). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes; Diabetes Care; 41(Suppl. 1): S13–S27.
- Arshad, R., Karim, N. and Hasan, J. A. (2014). Effects of insulin on placental, fetal and maternal outcomes in gestational diabetes mellitus. Pak J Med Sci;30(2):240-244.
- Bas-Lando, M., Srebnik, N., Farkash, R., et al. (2014). Elective induction of labor in women with gestational diabetes mellitus: an intervention that modifies the risk of cesarean section. Arch Gynecol Obstet;290(5):905-12.
- Berger, H., Gagnon, R. and Sermer, M. (2016). Diabetes in Pregnancy. JOGC; 38(7):667-679.e1.
- Biesty, L. M., Egan, A. M., Dunne, F., et al. (2018). Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No.: CD012910.
- Bomba-Opoń, D., Drews, K., Huras, H., et al. (2017). Polish Gynecological Society Recommendations for Labor Induction. Ginekol Pol; 88(4):224-234.
- Brown, J., Alwan, N. A., West, J., et al. (2017a). Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews, Issue 5. Art. No.: CD011970.
- Brown, J., Grzeskowiak, L., Williamson, K., et al. (2017b). Insulin for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews, Issue 11.
- Caughey (2018). UpToDate: GDM Obstetrical Issues and Management. Subscription required.
- Clausen, T. D., Mathiesen, E. R., Hansen, T., et al. (2009). Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab;94:2464–70.
- Declercq, E. R., Sakala, C., Corry, M. P., et al. (2013). Listening to MothersSM III: Pregnancy and Birth. New York: Childbirth Connection.
- Dublin, S., Johnson, K. E., Walker, R. L., et al. (2014). Trends in Elective Labor Induction for Six United States Health Plans, 2001-2007. Journal of Women’s Health. Vol. 23, No. 11.
- England, L. J., Dietz, P. M., Njoroge, T., et al. (2009). Preventing type 2 diabetes: public health implications for women with a history of gestational diabetes mellitus. Vol. 200, Issue: 365.e1-365.e8.
- Farrar, D., Simmonds, M., Bryant, M., et al. (2017). Treatments for gestational diabetes: a systematic review and meta-analysis. BMJ Open;7:e015557.
- Feghali, M. N., Caritis, S. N., Catov, J. M., et al. (2016). Timing of delivery and pregnancy outcomes in women with gestational diabetes. Am J Obstet Gynecol;215(2):243.e1-7.
- HAPO Study Cooperative Research Group (2008). Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002.
- Martis, R., Crowther, C. A., Shepherd, E., et al. (2018). Treatments for women with gestational diabetes mellitus: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No.: CD012327.
- Melamed, N., Ray, J. G., Geary, M., et al. (2016). Induction of labor before 40 weeks is associated with lower rate of cesarean delivery in women with gestational diabetes mellitus. Am J Obstet Gynecol;214:364.e1-8.
- Moyer, V. A. and U.S. Preventive Services Task Force (2014). Clinical Guideline: Screening for Gestational Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine; 160(6): 414-420.
- NICE guideline (2015). Diabetes in pregnancy: management from preconception to the postnatal period. Accessed October 19, 2018. Available online at: nice.org.uk/guidance/ng3.
- Niu, B., Lee, V. R., Cheng, Y. W., et al. (2014). What is the optimal gestational age for women with gestational diabetes type A1 to deliver?. Am J Obstet Gynecol;211(4):418.e1-6.
- Noordzij, M., van Diepen, M., Caskey, F. C., et al. (2017). Relative risk versus absolute risk: one cannot be interpreted without the other, Nephrology Dialysis Transplantation, Volume 32, Issue suppl_2, 1 April 2017, Pages ii13–ii18.
- Rosenstein, M. G., Cheng, Y. W., Snowden, J. M., et al. (2012). The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am J Obstet Gynecol 206: 309:e1–e7.
- Sutton, A. L., Mele, L., Landon, M. B., et al. (2014). Delivery timing and cesarean delivery risk in women with mild gestational diabetes mellitus. Am J Obstet Gynecol;211(3):244.e1-7.
- Tobias, D. K., Stuart, J. J., Shanshan, L., et al. (2017). Association of History of Gestational Diabetes with Long-Term Cardiovascular Disease Risk in Large Prospective Cohort of US Women. JAMA Internal Medicine. [Epub ahead of print].
- Vambergue, A. and Fajardy, I. (2011). Consequences of gestational and pregestational diabetes on placental function and birth weight. World J Diabetes; 2(11): 196-203.
- Witkop, C. T., Neale, D., Wilson, L. M., et al. (2009). Active compared with expectant delivery management in women with gestational diabetes: a systematic review. Obstet Gynecol;113:206–17.
Resources:
If you want to read more about induction or Cesarean for suspected big babies (with or without gestational diabetes), you can read this article.
Acknowledgments:
We would like to extend our gratitude to our expert reviewers: Shannon J. Voogt, MD, Board-Certified in Family Medicine; Courtney L. Barnes, MPH, MD, FACOG, specializes in Obstetrics & Gynecology in Columbia, MO; and Melissa Rosenstein, MD, Division of Maternal-Fetal Medicine at University of California, San Francisco.
We would also like to thank Cristen Pascucci for her medical editing assistance.
Birth Professionals:
Join others who also want to help bring evidence-based care to their local community.
Also, gain complimentary access to a printable library of our Signature Articles, 20+ hours of CE courses, a private community, and more.
Buy EBB Inspirational T-shirts, Due Date Buttons & Birth Affirmation Cards




Stay empowered, read more :
EBB 310 – Doulas & Nurses Advocating Together for Positive Shifts in Birth Culture with Joyce Dykema, EBB Instructor & Brianna Fields, RN
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | YouTube Dr. Rebecca Dekker is joined by Joyce Dykema, doula and EBB Instructor, and Brianna Fields, a labor and delivery nurse, to discuss advocating for positive shifts in...
EBB 308 – The Intersection of Environmental Justice and Midwifery Care with Dr. Tanya Khemet Taiwo
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | YouTube Curious which toxins should be most avoided for people of reproductive age and their children? In this episode, Dr. Tanya Khemet Taiwo, LM, CPM, MPH, PhD unravels the...
EBB 307 – Unexplained Infertility, Endometriosis, and a Birth Center Birth Story with Ellora La Shier, EBB Childbirth Class Graduate
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | GoogleIn this episode, Ellora La Shier, graduate of the EBB Childbirth Class, shares about her struggle with six years of unexplained infertility and how it impacted her...



