
Rebecca Dekker
PhD, RN
Evidence on: Induction or Cesarean for a Big Baby
This article was originally published in 2013 and last updated on July 21, 2021, by Rebecca Dekker, PhD, RN, and Erin Wilson, MPH. © Evidence Based Birth®, All Rights Reserved. Please read our Disclaimer and Terms of Use. For a printer-friendly PDF, become a Professional Member to access our complete library.
What is a big baby?
The medical term for big baby is macrosomia, which literally means “big body.” Some researchers consider a baby to be big when it weighs 4,000 grams (8 lbs., 13 oz.) or more at birth, and others say a baby is big if it weighs 4,500 grams (9 lbs., 15 oz.) or more (Rouse et al. 1996). Babies are called “extremely large” if they are born weighing more than 5,000 grams (11 lbs.) (Hehir et al. 2015).
A baby is also called “large for gestational age” if its weight is greater than the 90th percentile at birth, in other words, if it is bigger than 90% of all other babies born at that same gestational age (Rouse et al. 1996).
How common are big babies?
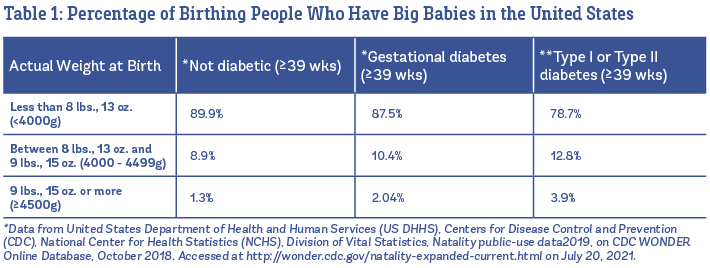
About one in ten babies is born big in the United States (U.S.). Overall, 8.9% of all babies born at 39 weeks or later weigh between 8 lbs., 13 oz., and 9 lbs., 15 oz., and 1.3% are born weighing 9 lbs., 15 oz. or more (U.S. Vital Statistics, 2019). In Table 1, you can see the percentages listed separately babies born to people who are not diabetic, vs. babies born to those with gestational diabetes and Type I or Type II diabetes.
What factors are linked to having big babies? Big babies run in families (this is influenced by genetics), and it’s more common to have a big baby when the baby’s sex is male (Araujo Júnior et al. 2017). As you can see in Table 1, people with diabetes before or during pregnancy have higher rates of big babies compared to people who are non-diabetic. Other factors that are linked to big babies include having a higher body mass index (BMI) before pregnancy, higher weight gain during pregnancy, older age, post term pregnancy, and a history of having a big baby (Araujo Jr. et al. 2017; Rui-Xue et al. 2019; Fang Fang et al. 2019).
Among people with gestational diabetes, researchers have found that having a higher blood sugar at first diagnosis makes you more likely to have a baby who is large for gestational age. (Metzger et al. 2008). However, pregnant people who manage their gestational diabetes through diet, exercise, or medication can bring down their chances of having a big baby to normal levels (or about 7%) (Landon et al. 2009).
In addition, there is high quality evidence from 15 randomized trials showing that pregnant parents who exercise (both those with and without diabetes) have a significant decrease in the chance of having a big baby compared to those who do not exercise during pregnancy (Davenport et al. 2018).
What is routine care for suspected big babies?
The most detailed evidence we have on typical care for big babies comes from the U.S. Listening to Mothers III Survey, which was published in the early 2010s. Although only one in ten babies is born large, researchers found that two out of three families in the U.S. had an ultrasound at the end of pregnancy to determine their baby’s size, and one out of three families in the study were told that their babies were too big. In the end, the average birth weight of their suspected “big babies” was only 7 lbs., 13 oz. (Declercq, Sakala et al. 2013).
Of the people who were told that their baby was getting big, two out of three said their care provider discussed inducing labor because of the suspected big baby, and one out of three said their care provider talked about planning a Cesarean because of the big baby.
Most of the families whose care providers talked about induction for big baby ended up being medically induced (67%), and the rest tried to self-induce labor with natural methods (37%). Nearly one in five survey respondents said they were not offered a choice when it came to induction—in other words, they were told that they must be induced for their suspected big baby.
When care providers brought up planning a Cesarean for a suspected big baby, one in three families ended up having a planned Cesarean. Two out of five survey respondents said that the discussion was framed as if there were no other options—that they must have a Cesarean for their suspected big baby.
In the end, care provider concerns about a suspected big baby were the fourth most common reason for an induction (making up 16% of all inductions), and the fifth most common reason for a Cesarean (making up 9% of all Cesareans). More than half of all birthing people (57%) believed that an induction was medically necessary if a care provider suspects a big baby.
So, in the U.S., most people have an ultrasound at the end of pregnancy to estimate the baby’s size, and if the baby appears large, their care provider will usually recommend either an induction or an elective Cesarean. Is this approach evidence-based?
This approach is based on five major assumptions:
- Big babies have a higher risk of their shoulders getting stuck (also known as shoulder dystocia).
- Big babies are at higher risk for other birth problems.
- We can accurately tell if a baby will be big.
- Induction keeps the baby from getting any bigger, which lowers the risk of Cesarean.
- Elective Cesareans for big baby are only beneficial; that is, they don’t have major risks that could outweigh the benefits.
Assumption #1: Big babies are at higher risk for getting their shoulders stuck (shoulder dystocia).
Reality #1: While it is true that 7-15% of big babies have difficulty with the birth of their shoulders, most of these cases are handled by the care provider without any harmful consequences for the baby. Permanent nerve injuries due to stuck shoulders happen in 1 out of every 555 babies who weigh between 8 lbs., 13 oz. and 9 lbs., 15 oz., and 1 out of every 175 babies who weigh 9 lbs., 15 oz. or greater.
One of the main concerns with big babies is shoulder dystocia (“dis toh shah”). Shoulder dystocia is defined as when shoulders are stuck enough that the care provider has to take extra physical action(s), or maneuvers, to help get the baby out.
In the past, researchers have referred to shoulder dystocia as the “obstetrician’s greatest nightmare” (Chauhan 2014). The fear with shoulder dystocia is that it is possible that the baby might not get enough oxygen if the head is out but the body does not come out shortly afterwards. There is also a risk that the baby will experience a permanent nerve injury to the shoulders.
One of the reasons that care providers have a fear of shoulder dystocia is because if the baby experiences an injury during or after shoulder dystocia, this type of injury is a common cause of litigation. In a study carried out at the University of Michigan, researchers found that half of all parents whose children were being treated for shoulder dystocia-related injuries were pursuing litigation (Domino et al. 2014).
How often does shoulder dystocia occur? Researchers who combined results from ten studies found that shoulder dystocia happened to 6% of babies who weighed more than 4,000 grams (8 lbs., 13 oz.) versus 0.6% of those who were not big babies (Beta et al. 2019). When babies weighed more than 4,500 grams (9 lbs., 15 oz.), 14% experienced shoulder dystocia.
Similarly, one high-quality study that looked separately at pregnant people with and without diabetes showed that in non-diabetic people, shoulder dystocia happened to 0.65% of babies who weighed less than 8 lbs., 13 oz. (6.5 cases out of 1,000 births), 6.7% of babies who weighed between 8 lbs., 13 oz. and 9 lbs., 15 oz. (60 out of 1,000), and 14.5% of babies who weighed 9 lbs., 15 oz. or greater (145 out of 1,000) (Rouse et al. 1996).
Rates of shoulder dystocia were much higher in big babies whose birthing parent had Type I and Type II diabetes (2.2% of babies that weighed less than 8 lbs., 13 oz., 13.9% of babies that weighed between 8 lb., 13 oz. and 9 lb., 15 oz., and 52.5% of babies that weighed more than 9 lb., 15 oz.) (Rouse et al. 1996).
We were not able to find exact numbers for the percentage of people with gestational diabetes who had a baby with shoulder dystocia, as the rates change depending on each person’s blood sugar level. However, there is strong evidence that treatment for gestational diabetes drastically lowers the chance of having a big baby and shoulder dystocia. We cover the evidence on treatment for gestational diabetes (link evidencebasedbirth.com/inducingGDM) in our Evidence Based Birth® Signature Article on Induction for Gestational Diabetes.
It’s interesting to note that people with high blood sugar levels during pregnancy are at increased risk of shoulder dystocia during birth even when the baby is not big. This is because weight can be distributed differently on a baby when their gestational carrier has high blood sugars. Problems are more likely to occur if the baby’s head size is relatively small compared to the size of its shoulders and abdomen (Kamana et al. 2015).
Although big babies are at higher risk for shoulder dystocia, at least half of all cases of shoulder dystocia happen in smaller or normal sized babies (Morrison et al. 1992; Nath et al. 2015). This is because overall, there are more small and normal size babies born than big babies. In other words, the rate of shoulder dystocia is higher in bigger babies, but the absolute numbers are about the same with bigger and smaller babies. Unfortunately, researchers have found that it is impossible to predict exactly who will have shoulder dystocia and who will not (Foster et al. 2011).
Because at least half of shoulder dystocia cases occur in babies that are not big, and we can’t predict who will have a shoulder dystocia, shoulder dystocia will always be a possibility during childbirth. That is, the risk can only be eliminated if all babies are born by Cesarean. Because requiring everyone to have a Cesarean is unethical and impractical, it is important for health care providers to train for the possibility of a shoulder dystocia.
Other resources on resolving shoulder dystocia:
- There are ways care providers can help prevent and manage a shoulder dystocia. For more information, read this article on shoulder dystocia by Midwife Thinking.
- Click here for a PowerPoint from a shoulder dystocia training course from the United Kingdom.
- Spinning Babies offers an online continuing education course about resolving shoulder dystocia. You can also download a free PDF on the FLIP-FLOP technique for managing shoulder dystocia here.
- This video and this article describe how care providers can use a technique called the “shoulder shrug maneuver” to resolve shoulder dystocia (Sancetta et al. 2019).
- The Royal College of Obstetricians and Gynecologists has a guideline (last reviewed in 2017) on predicting, preventing, and managing shoulder dystocia here.
Brachial plexus palsy
A shoulder dystocia by itself is not considered a “bad outcome.” It’s only a bad outcome if an injury occurs along with the shoulder dystocia (Personal communication, Emilio Chavirez, MD, FACOG, FSMFM). Although most cases of shoulder dystocia can be safely managed by a care provider during the birth, some can result in a nerve injury in the baby called brachial plexus palsy.
Brachial plexus palsy, which leads to weakness or paralysis of the arm, shoulder, or hand, happens in about 1.3 out of every 1,000 vaginal births in the U.S. and other countries. A baby does not have to have shoulder dystocia to experience a brachial plexus palsy—in fact, 48%-72% of brachial plexus palsy cases happen without shoulder dystocia. When a brachial plexus palsy happens at the same time as shoulder dystocia, however, it is more likely to end up in a lawsuit than a brachial plexus palsy that did not occur with a shoulder dystocia (Chauhan et al. 2014).
Although rare, brachial plexus palsy can also happen to babies born by Cesarean. In one study that looked at 387 children who experienced brachial plexus palsy, 92% were born vaginally and 8% were born by Cesarean (Chang et al. 2016). Other researchers have found that brachial plexus palsy happens in about 3 per 10,000 Cesarean births (Chauhan et al. 2014).
Some infants who have a brachial plexus palsy (about 10%-18%) will end up with a permanent injury, defined as arm or shoulder weakness that persists for more than a year after birth. It’s estimated that there are anywhere from 35,000 to 63,000 people living with permanent brachial plexus injuries in the U.S. (Chauhan et al. 2014). For a blog article about what it’s like to grow up with a brachial plexus palsy, read Nicola’s story here.
In 2019, researchers combined five studies about the risks of brachial plexus injury in pregnancies with babies over 8 lbs., 13 oz. versus those with babies who were not big (Beta et al 2019). Big babies had significantly more brachial plexus injury (0.74% versus 0.06%). When babies weighed more than 4,500 grams (9 lbs., 15 oz.), the rate went up to 1.9%.
In a recent study of infants who were all extremely large at birth (>5000 g, or >11 lbs.), 17 of 120 infants born vaginally had shoulder dystocia, and three of those 17 had temporary brachial plexus palsy that healed within six months—for an overall rate of about 1 brachial plexus palsy cases per 40 vaginally-born, extremely large babies (Hehir et al. 2015).
In 1996, Rouse et al. published rates of shoulder dystocia and brachial plexus palsy by infant weight. Using the numbers of permanent disability published by Chauhan et al. in 2014, we created a table that helps show the difference between the weight groups.

Importantly, research has shown that when health care professionals undergo annual inter-professional training (this means doctors, nurses, and midwives training together as a team) on how to handle shoulder dystocia, they can lower—and in some cases eliminate—brachial plexus palsy among babies who experience shoulder dystocia (Crofts et al. 2016). Doctors have been trying to take this successful training (called “PROMPT”) from the United Kingdom and implement it in the U.S. Results at the University of Kansas showed a decline and then an eventual elimination of permanent cases of brachial plexus palsy with PROMPT annual trainings (Weiner et al. 2015).
To watch a news video about the PROMPT training, click here. To visit the PROMPT foundation website, clickhttps://www.promptmaternity.org/.
Can a baby die from shoulder dystocia?
Deaths from shoulder dystocia are possible but rare. In 1996, researchers looked at all the studies so far that had reported the rate of death due to shoulder dystocia. In 15 studies, there were 1,100 cases of shoulder dystocia and no deaths (a death rate of 0%). In two other studies, the rates of infant death were 1% (one baby out of 101 “died at delivery,” possibly due to the shoulder dystocia) and 2.5% (one infant died out of 40 cases of shoulder dystocia) (Rouse et al. 1996).
In a study published by Hoffman et al. in 2011, researchers looked at 132,098 people who gave birth at term to a live baby in head- first position. About 1.5% of the babies had a shoulder dystocia (2,018 cases), and of those, 101 newborns were injured. Most of the injuries were brachial plexus palsy or collar bone fractures. Out of the 101 injured infants, there were zero deaths and six cases of brain damage due to lack of oxygen. With the six brain-damaged infants, it took an average of 11 minutes between the birth of the head and the body.
Assumption #2: Big babies can lead to a higher risk of health problems and complications.
Reality #2: The risk of complications with a big baby increases along a spectrum (lower risk at 8 lbs., 13 oz., higher risk at 9 lbs., 15 oz., and highest risk at 11+ lbs.). In addition, the care provider’s “suspicion” of a big baby carries its own set of risks.
Unplanned Cesareans
Researchers combined 10 studies (called a meta-analysis) and found that babies with birth weights over 4,000 grams (8 lbs., 13 oz.) are more likely to have labors that end in Cesarean (Beta et al. 2019). In these studies, the average Cesarean rate was 19.3% for big babies versus 11.2% for babies who were not big. When babies weighed more than 4,500 grams (9 lbs., 15 oz.), the Cesarean rate increased to 27%. As we will discuss, a care provider’s “suspicion” of a big baby can impact their likelihood of recommending Cesarean during labor.
Perineal Tears
In the meta-analysis published by Beta et al. (2019), five studies found a significant increase in the odds of severe tears with big babies, while three studies did not find a difference. When the researchers combined the results from all eight studies, the overall result showed that those who give birth to big babies are more likely to have severe perineal tears, also known as 3rd or 4th degree tears. The risk of a severe tear was 1.7% when birthing big babies versus 0.9% for birthing babies who were not big. When babies weighed more than 4,500 grams (9 lbs., 15 oz.), the rate of severe tears was 3%.
The largest study (over 350,000 pregnant participants from National Health Service hospitals) examined 3rd degree tears and found the rate to be 0.87% with big babies versus 0.45% without (Jolly et al. 2003). In this study, pregnancies with big babies were also more likely to have longer first and second stages of labor and more use of vacuum and forceps. The increase in the use of vacuum and forceps among big babies likely contributed to the increase in severe tears.
The second largest study, which included over 146,000 hospital births in California between 1995 and 1999, found a higher rate of 4th degree tears in big babies who were born vaginally (Stotland et al. 2004). However, 4th degree tear rates in this study were very high, even among normal weight babies (1.5%), and the authors did not describe how many birthing people had episiotomies, which is a leading cause of severe tears.
Although having a big baby may be a risk factor for severe tears, it may be helpful to compare this risk to other situations that can also increase the risk of tears. For example, one large study found that the risk of a severe tear with a big baby ranged from 0.2% to 0.6% (Weissmann-Brenner et al. 2012).Other researchers have found that a vacuum delivery increases the risk of a severe tear by 11 times. So, if your baseline risk was 0.2%, it would increase to 2.2% with a vacuum, and the use of forceps increases the risk of a severe tear by 39 times (from 0.2% to 7.8%) (Sheiner et al. 2005).
Postpartum Hemorrhage
Researchers combined nine studies that reported on postpartum hemorrhage in people who gave birth to big babies compared to those who birthed babies who were not big (Beta et al. 2019). They found a higher rate of hemorrhage with babies over 8 lbs., 13 oz. (4.7% versus 2.3%). When the birth weights were over 4,500 grams (9 lbs., 15 oz.), the rate of postpartum hemorrhage was 6%. However, it is not clear whether this higher rate of postpartum hemorrhage is due to the big babies themselves or the inductions and Cesareans that care providers often recommend for a suspected big baby (Fuchs et al. 2013)—as both these procedures can increase the risk of postpartum hemorrhage (Magann et al. 2005).
Newborn complications
One study compared 2,766 large babies with the same number of babies with normal birth weights. All babies in the study were born to non-diabetic parents (Linder et al. The researchers found that big babies were more likely to have low blood sugar after birth (1.2% vs. 0.5%), temporary rapid breathing (also known as “transient tachypnea” or “wet lung,” 1.5% vs. 0.5%), high temperature (0.6% vs. 0.1%), and birth trauma (2% vs. 0.7%).
The researchers did not say whether care providers suspected that the babies were large before labor began, or if their care was managed differently. More of the large infants in this study were born by Cesarean (33% vs. 15%), which could have played a role in the higher rates of breathing problems, since breathing problems are more common with Cesarean-born babies.
Birth fractures, or broken collar bones or arms, are rare but more likely to occur among big babies. Researchers combined the results from five studies and found that the rate of birth fractures among babies over 4,000 grams (8 lbs., 13 oz.) was 0.54% versus 0.08% among babies who are not big (Beta et al. 2019). When babies weighed more than 4,500 grams (9 lbs., 15 oz.), the fracture rate was increased to 1.01%.
Stillbirth
Some doctors recommend Cesareans for suspected big babies because they believe there is a higher risk of stillbirth.
In 2014, researchers published a study where they looked back in time at 784,576 births that took place in Scotland between the years 1992 and 2008. They included all babies who were born at term or post-term (between 37 and 43 weeks). They did not include multiples or any babies who died from congenital anomalies (Moraitis et al. 2014).
Babies in this study were grouped according to their size for gestational age—4th to 10th percentile, 11th to 20th percentile, 21st to 80th percentile (considered the normal group), 81st to 90th percentile, 91st to 97th percentile, and 98th to 100th percentile. The gestational age of each baby was confirmed by ultrasounds that took place in the first half of pregnancy.
In this study, there were 1,157 stillbirths, and the risk of stillbirth was highest in the groups with the smallest babies (1st to 3rd and 4th to 10th percentiles). The third highest risk of stillbirth death was seen in the babies who were in the 98th to 100th percentiles for weight (extremely large for gestational age). Using the American Academy of Pediatrics growth curve for gestational age, the 98th to 100th percentiles would be roughly equivalent to a baby who is born weighing 9 lbs., 15 oz. or greater at 41 weeks.
Meanwhile, the lowest rates of stillbirth were in babies who were in the 91st to 97th percentiles. The increase in stillbirth risk in the largest group (98th to 100th percentile) was partly explained by the birth parent being diabetic; however, there was also a higher risk of unexplained stillbirth for babies in the 98th to 100th percentile. Overall, the absolute risk of an extremely large for gestational age baby (98th to 100th percentile) experiencing stillbirth between 37 and 43 weeks was about 1 in 500, compared to 1 in 1,000 for babies who are in the 91st to 97th percentile.
Another study on this topic looked back in time at 693,186 births and 3,275 stillbirths between 1992-2009 in Alberta, Canada (Wood and Tang, 2018). They included all babies born at ≥23 weeks but did not include multiples.
This large Canadian database study found several risk factors for stillbirth: giving birth for the first time, having higher body mass index (BMI), smoking in pregnancy, older age, and having medical problems before pregnancy such as high blood pressure and diabetes. Like the previous study, small for gestational age was a strong risk factor for stillbirth. But babies who were large for gestational age were not at any increased risk for stillbirth. In fact, being large for gestational age was protective against stillbirth in the general population.
However, when researchers looked specifically at birth parents with gestational diabetes, being large for gestational age was linked with a higher risk of stillbirth. The same was true for birth parents with Type I or Type II diabetes.
The risk of stillbirth has historically been higher in pregnant people with Type I or Type II diabetes. However, in recent years the stillbirth rate for those with Type I or Type II diabetes has drastically declined due to improvements in how diabetes is managed during pregnancy (Gabbe et al. 2012). As far as gestational diabetes goes, the largest study ever done on gestational diabetes found no link between gestational diabetes and stillbirth (Metzger et al. 2008). In the Canadian study, gestational diabetes was not linked with a higher risk of stillbirth unless the baby was also considered to be large for gestational age.
In 2019, a large study in the U.S. analyzed medical records of stillbirths that occurred between 1982 and 2017. The purpose of this study was to look at the possible relationship between big babies and stillbirth, but other factors were also considered (Salihu et al. 2014). It is important to note that overall, the rates of stillbirth have declined dramatically in both big and normal-sized babies over the last four decades. The decline in stillbirths may be due to advancements in medical training and pregnancy screening. In this study population, the rate of stillbirth in big babies declined 48.5% (from 2.04 per 1,000 to 1.1 per 1000), and it also declined 57.4% in babies of normal size (from 1.95 per 1,000 to 0.83 per 1000).
In total, more than 100 million pregnancies were analyzed in this study. About 10% of the total number of pregnancies were big babies. In the big baby group, there were 1.2 stillbirths per 1,000 pregnancies, compared to 1.1 stillbirths per 1,000 pregnancies in the normal birth weight range.
The researchers point out that the risk of a big baby being stillborn varies from situation to situation, and so care should be individualized. In other words, not all big babies carry the same level of potential risk when it comes to the chances of stillbirth. In their study, researchers separated the babies into 3 groups (grade 1 or 4000-4499 grams, grade 2 or 4500-5000 grams, and grade 3 or over 5000 grams). Babies in the grade 3 group experienced an 11-fold increase in stillbirth (11 stillbirths per 1,000 pregnancies) when compared to babies in the grade 1 group (1 stillbirth per 1,000 pregnancies). However, grade 3 big babies made up only 1.5% of the total big baby group, while grade 1 big babies made up more than 85% of the total big baby group. Overall, the group with the highest risk of stillbirth was the low birthweight group (14.89 stillbirths per 1,000 pregnancies). The second highest rate of stillbirth was in the grade 3 big baby group. Some strengths of this study are the large data set and the classification of big babies into grades of macrosomia. A limitation is that because of the way the data was collected, we don’t know if pregnant people who were diagnosed with “diabetes” had gestational diabetes or pre-existing Type 1 or Type 2 diabetes.
Is it Harmful to Suspect a Big Baby?
When a big baby is suspected, families are more likely to experience a change in how their care providers see and manage labor and birth. This leads to a higher Cesarean rate and a higher rate of people inaccurately being told that labor is taking “too long” or the baby “doesn’t fit.”
In fact, research has consistently shown that the care provider’s perception that a baby is big can be more harmful than an actual big baby by itself.
Overall, a total of nine different studies from 1992 to 2015 have all shown that it is the suspicion of a big baby—not big babies themselves—that leads to higher induction rates, higher Cesarean rates, and higher diagnoses of stalled labor (Levine et al., 1992; Weeks et al., 1995; Parry et al., 2000; Weiner et al., 2002; Sadeh-Mestechkin et al., 2008; Blackwell et al., 2009; Melamed et al., 2010; Little et al., 2012; Peleg et al., 2015).
In one study, researchers compared what happened when people were suspected of being pregnant with a big baby (>8 lbs., 13 oz.) versus people who were not suspected of being pregnant with a big baby—but who ended up having one (Sadeh-Mestechkin et al. 2008).
The end results were astonishing. Birthing people who were suspected of having a big baby (and actually ended up having one) had triple the induction rate, more than triple the Cesarean rate, and a quadrupling of the maternal complication rate, compared to those who were not suspected of having a big baby but had one anyway.
Complications were most often due to Cesareans and included bleeding (hemorrhage), wound infection, wound separation, fever, and need for antibiotics. There were no differences in shoulder dystocia between the two groups. In other words, when a care provider “suspected” a big baby (as compared to not knowing the baby was going to be big), this tripled the Cesarean rates and made mothers more likely to experience complications, without affecting the rate of shoulder dystocia (Sadeh-Mestechkin et al. 2008).
These results were supported by another study published by Peleg et al. in 2015. At their hospital, physicians had a policy to counsel everyone with suspected big babies (suspected of being 8 lbs., 13 oz. and higher, or ≥4,000 grams) about the “risks” of big babies. Elective Cesareans were not encouraged, but they were performed if the family requested one after the discussion. There were 238 participants who had suspected big babies (that ended up truly being large at birth) and were counseled, and 205 participants who had unsuspected big babies (that ended up being truly large at birth) who were not counseled.
Even though the babies were all about the same size, only 52% of participants in the suspected big baby group had a vaginal birth, compared to 91% of participants in the non-suspected big baby group. This increase in Cesarean rate in the suspected big baby group was primarily due to an increase in the families requesting elective Cesareans after the “counseling” session about how big babies are risky to birth. There was only one case of shoulder dystocia in the unsuspected big baby group, and two cases in the suspected big baby group. None of these babies experienced injuries. There was no difference in severe birth injuries between the two groups.
The authors concluded that obstetricians should not be counseling pregnant people about the risks of big babies thought to be 8 lbs. 13 oz. or higher, because it leads to an increase in the number of unnecessary Cesareans without any benefit to the birthing person or baby. They suggested that researchers should study using a higher weight cut-off (such as 9 lbs., 15 oz.) to trigger counseling.
Other researchers have found that when a first-time parent is incorrectly suspected of having a big baby, care providers have less patience with labor and are more likely to recommend a Cesarean for stalled labor. In this study, researchers followed 340 first-time birthing people who were all induced at term. They compared the ultrasound estimate of the baby’s weight with the actual birth weight. When the ultrasound incorrectly said the baby was going to weigh more than 15% higher than it ended up weighing at birth, physicians were more than twice as likely to diagnose “stalled labor” and perform a Cesarean for that reason (35%) than if there was no overestimation of weight (13%) (Blackwell et al. 2009b).
Pregnant people who are plus size and those who take medication for high blood sugar also experience an increase in unplanned Cesareans when ultrasound is used to estimate the baby’s weight (Dude et al. 2019; Dude et al. 2018).
A recent study from the U.S. looked at 2,826 first-time birthing people with a body mass index (BMI) ≥ 35 kg/m2 (Dude et al. 2019). Out of everyone in the study, 23% had an ultrasound to estimate the baby’s weight within 35 days of birth. The participants who had an ultrasound to estimate the baby’s weight were more likely to have an unplanned Cesarean (mostly for “stalled labor”) than those without an ultrasound-estimated fetal weight (43% versus 30%). Having an ultrasound to estimate the baby’s weight was linked with a higher rate of Cesarean even after considering other factors that could have impacted the Cesarean rate, including the baby’s actual birth weight.
Among the 636 participants who had an ultrasound to estimate the baby’s weight, 143 of them were told that their babies were large for gestational age (measuring over the 90th percentile). This group had a much higher rate of Cesarean (61% versus 31%). However, only 44% of them (61 out of the 143 birthing people) gave birth to a baby that was large for gestational age.
The authors found similar results when they looked at around 300 people who were giving birth for the first time and taking medication for high blood sugar (Dude et al. 2018). Again, having an ultrasound to estimate the baby’s weight within 35 days of birth was linked to a higher rate of unplanned Cesareans (52% for those with an ultrasound versus 27% for those without an ultrasound) even after considering the baby’s actual birth weight and other medical factors.
The authors conclude, “Perceived knowledge of fetal weight may affect decisions providers make regarding how likely they feel their patients are to deliver vaginally.”
It’s not surprising that physicians are more likely to turn to Cesarean in these situations, given a cultural fear of big babies. In one medical journal editorial, an obstetrician with a clear bias towards Cesarean for big babies said that, “Flagging up all cases of predicted fetal macrosomia is vitally important, so that the attendants in the labor suite will recommend Cesarean if there is any delay in cervical dilatation or arrest of head rotation or descent. Cesarean should also be the preferred option if an abnormal fetal heart tracing develops” (Campbell, 2014).
So, in summary, although big babies are at higher risk for some problems, the care provider’s perception that there is a big baby carries its own set of risks. This perception—whether it is true or false—changes the way the care provider behaves and how they talk to families about their ability to birth their baby, which, in turn, increases the chance of Cesarean.
Assumption #3: We can tell which babies will be big at birth.
Reality #3: Both physical exams and ultrasounds are equally bad at predicting whether a baby will be big at birth.
Time and time again, researchers have found that it is very difficult to predict a baby’s size before it is born. Although two out of three people giving birth in the U.S. receive an ultrasound at the end of pregnancy (Declercq et al. 2013) to “estimate the baby’s size,” both the care provider’s estimate of the baby’s size and ultrasound results are unreliable.
In 2005, researchers looked at all the studies that had ever been done on ultrasound and estimating the baby’s weight at the end of pregnancy. They found 14 studies that looked at ultrasound and its ability to predict that a baby would weigh more than 8 lbs., 13 oz. Ultrasound was accurate 15% to 79% of the time, with most studies showing that the accuracy (“post-test probability”) was less than 50%. This means that for every ten babies that ultrasound predicts will weigh more than 8 lbs., 13 oz., five babies will weigh more than that and the other five will weigh less (Chauhan et al. 2005).
Ultrasound was even less accurate at predicting babies who will be born weighing 9 lbs., 15 oz. or greater. In three studies that were done, the accuracy of ultrasounds to predict extra-large babies was only 22% to 37%. This means that for every ten babies the ultrasound identified as weighing more than 9 lbs., 15 oz., only two to four babies weighed more than this amount at birth, while the other six to eight babies weighed less (Chauhan et al. 2005).
The researchers found three studies that looked at the ability of ultrasound to predict big babies in pregnant people with diabetes. The accuracy of these ultrasounds was 44% to 81%, which means that for every ten babies of a diabetic parent who are thought to weigh more than 8 lbs., 13 oz., around six will weigh more and four will weigh less. The ultrasound test probably performs better in diabetics simply because diabetics are more likely to have big babies. In other words, it’s easier to predict a big baby in someone who is much more likely to have a big baby to begin with.
Currently, there is no reason to believe that three-dimensional (3D) ultrasound is any better at predicting birth weight and big babies than two-dimensional (2D) ultrasound (Tuuli et al. 2016). Research is ongoing to determine if 3D measurements can be combined with 2D measurements to better predict macrosomia.
There is also no evidence that magnetic resonance imaging (MRI) improves the accuracy of fetal weight estimates. The first prospective clinical study to compare estimated fetal weight from 2D ultrasound versus MRI is currently being conducted in Belgium (Kadji et al. 2019). The researchers think that MRI at 36 to 37 weeks of pregnancy could be much more accurate than ultrasound at predicting big babies. However, even if MRI is found to be superior, it is very expensive and probably not practical.
Compared to using ultrasound, care providers are just as inaccurate when it comes to using a physical exam to estimate the size of the baby. However, ultrasound appears to provide more accurate estimates when pregnant people are plus size (Preyer et al. 2019).
Overall, when a care provider estimates that a baby is going to weigh more than 8 lbs., 13 oz., the accuracy is only 40-53% (Chauhan et al. 2005). This means that out of all the babies that are thought to weigh more than 8 lbs., 13 oz., half will weigh more than 8 lbs., 13 oz. and half will weigh less.
The care provider’s accuracy goes up if the pregnant person has diabetes or is post-term, again, probably because the chance of having a big baby is higher among these groups. Unfortunately, all the studies that looked at diabetes and the accuracy of ultrasound lumped people with gestational diabetes and those with Type I or Type II diabetes into the same groups, limiting our ability to interpret these results.
A systematic review concluded that there is “no clear consensus with regard to the prenatal identification, prediction, and management of macrosomia.” The authors stated that the main problem with big babies is that it is very difficult to diagnose big babies before birth—it’s a diagnosis that can only be made after birth (Rossi et al. 2013).
Even the “best” way to predict a big baby is going to have problems identifying actual big babies—most often overestimating the size of the baby. In a 2010 study by Rosati et al., researchers tested different ultrasound “formulas” to figure out an infant’s estimated weight. The best formula for predicting birth weight was the “Warsof2” formula, which is based solely on the baby’s abdominal measurement. The results of this formula came within ±15% of the baby’s actual weight in 98% of cases. As an example, if your baby’s actual weight was 8 lbs. (3,629 grams), the ultrasound could estimate the baby’s weight to be anywhere between 6 lbs., 13 oz. (3,090 grams) and 9 lbs., 3 oz. (4,450 grams).
Many weight estimation formulas have been published (new 2D and 3D formulas are added every year), and researchers continue to debate whether they are accurate.
Recently, a study compared the “Hart” weight estimation formula to the “Hadlock” formula (Weiss et al. 2018). The “Hadlock” formula is very popular today and considered by many to be the most accurate (Milner and Arezina, 2018). Weiss et al. found that compared to the “Hadlock” formula, the “Hart” formula greatly overestimated fetal weight when babies weighed less than 8 lbs., 13 oz. (4,000 grams) and failed to detect very large babies. The authors expressed concern that using the “Hart” formula could lead to an increased rate of labor induction and Cesareans, and they concluded that it has no place in clinical practice.
Assumption #4: Induction allows the baby to be born at a smaller weight, which helps avoid shoulder dystocia and lowers the risk of Cesarean.
Reality #4: There is conflicting evidence about whether induction for suspected big babies can improve health outcomes.
We will talk about three main pieces of evidence in this section:
- A 2016 Cochrane review (when researchers combined multiple randomized trials together)
- The largest study (published in 2015) from the Cochrane review
- The second-largest study (published in 1997) from the Cochrane review
Cochrane Review
In a 2016 Cochrane review, researchers (Boulvain et al. 2016) combined four studies in which 1,190 non-diabetic pregnant people with suspected big babies were randomly assigned (like flipping a coin) to either 1) induction between 37 and 40 weeks or 2) waiting for spontaneous labor.
When researchers compared the induction group to the waiting group, they found a decrease in the rate of shoulder dystocia in the induction group—about 41 cases per 1,000 births in the elective induction group, down from 68 cases per 1,000 in the waiting group.
They also found a decrease in birth fractures in the elective induction group (4 per 1,000 vs. 20 per 1,000 in the waiting group). To prevent one fracture, it would be necessary to induce labor in 60 people.
On the other hand, they found an increase in severe perineal tears in the induction group (26 per 1,000 in the induction group vs. 7 per 1,000 in the waiting group), as well as an increase in the treatment of jaundice (11% vs. 7%).
On average, babies weighed 178 grams (6 ounces) less when labor was electively induced, compared with those assigned to wait for labor.
There were no differences between groups in rates of Cesarean, instrumental delivery, NICU admissions, brachial plexus palsy, or low Apgar scores. Three of the four studies reported death rates, and there were zero deaths in either group.
Researchers did not look at patients’ satisfaction with their care or any long-term health results for birthing people or babies.
Largest study in Cochrane review (2015)
The study published by Boulvain et al. 2015 was the largest study in the Cochrane review. In this study, researchers followed 818 pregnant people with suspected big babies who were randomly assigned to either a) induce labor between 37 to 38 weeks, or b) wait for labor to start on its own until 41 weeks. This is the largest randomized trial that has ever been done on induction for suspected big babies.
Pregnant people could be in the study if they had a single baby in head-down position, whose estimated weight was in the 95th percentile (>7 lbs., 11 oz. at 36 weeks, 8 lbs., 3 oz. at 37 weeks, or 8 lbs., 10 oz. at 38 weeks). About 10% of the participants in this study had gestational diabetes.
There was some cross-over between groups: 11% of participants in the induction group went into labor on their own, and 28% of participants in the waiting-for-labor group were induced.
The researchers found that pregnant people randomly assigned to the induction group (whether or not they were actually induced) had fewer cases of shoulder dystocia: 1% of people in the induction group (5 out of 407) had true shoulder dystocia compared with 4% (16 out of 411) of those in the expectant management group. None of the babies in either group experienced any brachial plexus palsy injuries, and collarbone fracture rates were low in both groups (1 to 2%).
The chances of having a spontaneous vaginal birth was slightly more common in the induction group (59% vs. 52%), but there was no difference in the rates of Cesarean and the use of forceps or vacuum. There were no other differences in birth outcomes, including any tears or hemorrhage.
The infants in the induction group were more likely to have jaundice (9% vs. 3%) and receive phototherapy treatment (11% vs. 7%). There were no differences in NICU admission rates or any other newborn differences between groups.
In summary, this study found that early induction (at 37-38 weeks) lowered the rate of shoulder dystocia, but without any accompanying impact on actual brachial plexus palsy rates, collarbone fractures, or NICU admissions.
The authors suggested that the main reason they found different results from an earlier randomized trial by Gonen et al. (1997), is because they checked fetal weight earlier and induced babies earlier— between 37 to 39 weeks, instead of waiting until 38 to 39 weeks. This meant that they induced labor when a fetus is large for gestational age, but before it was technically “big,” resulting in the birth of a normally sized baby a few weeks early. For example, in the Gonen et al. study discussed next, pregnant people were not included in the study until they were at least 38 weeks pregnant and their estimated fetal weight reached 8 lbs., 13 oz. Meanwhile, in the newer trail by Boulvain et al., of the 411 infants in the waiting-for-labor group, 62% weighed more than 4000 g (8 lbs., 13 oz.) at birth, compared with 31% of those who were induced. This means that the participants who waited for labor to start on its own ended up with big babies, while those who were induced early gave birth before their babies could become large.
The authors of the Boulvain study think that previous studies have not found a benefit to induction because providers waited too long to intervene, and they missed their chance for the mother to birth a smaller baby and reduce the risk of shoulder dystocia. Although this approach—inducing labor between 37 and 39 weeks—resulted in lower rates of shoulder dystocia, it also led to higher rates of newborn jaundice, and it did not have any impact on “hard” outcomes such as brachial plexus palsy or NICU admission.
Second-largest study in the Cochrane Review
The Gonen et al. (1997) study was the second-largest study in the Cochrane review (with 273 participants). In this study, pregnant people were included if they were at least 38 weeks, had a suspected big baby (8 lbs., 13 oz. to 9 lbs., 15 oz.), did not have gestational diabetes, and had not had a previous Cesarean. Less than half the participants were giving birth for the first time. Participants were randomly assigned to either immediate induction with oxytocin (sometimes also with cervical ripening) or waiting for spontaneous labor.
The results? Participants in the spontaneous labor group went into labor about five days later than those who were immediately induced. Although participants in the spontaneous labor group tended to have slightly bigger babies (on average, 3.5 oz. or 99 grams heavier), there was no difference in shoulder dystocia or Cesarean rates. All 11 cases of shoulder dystocia, spread across both groups, were easily managed without any nerve damage or trauma. Two infants in the waiting-for-labor group had temporary and mild brachial plexus palsy, but neither of these two infants had shoulder dystocia. Finally, ultrasound overestimated the baby’s weight 70% of the time and under-estimated the baby’s weight 28% of the time.
In summary, the researchers found that: 1) ultrasound estimation of weight was inaccurate, 2) shoulder dystocia and nerve injury were unpredictable, and 3) induction for big baby did not decrease the Cesarean rate or the risk of shoulder dystocia.
Assumption #5: Elective Cesarean for big baby has benefits that outweigh the potential harms.
Reality #5: No researchers have ever carried out a study to determine the effects of elective Cesareans for suspected big babies.
Although some care providers will recommend an induction for a big baby, many skip this step and go straight to recommending an elective Cesarean. However, researchers have estimated that this type of approach is extremely expensive and that it would take thousands of unnecessary Cesareans to prevent one case of permanent brachial plexus palsy.
In 1996, an important analysis published in the Journal of the American Medical Association proposed that a policy of elective Cesareans for all suspected big babies was not cost-effective and that there were more potential harms than potential benefits (Rouse et al. 1996).
In this analysis, the researchers calculated the potential effects of three different types of policies:
- No routine ultrasounds to estimate the babies’ sizes
- Routine ultrasounds, then elective Cesarean for babies weighing 8 lbs., 13 oz. or more
- Routine ultrasounds, then elective Cesarean for babies weighing 9 lbs., 15 oz. or more
The researchers looked at the results separately for diabetic and non-diabetic people. Unfortunately, most research up to this time point did not distinguish between Type 1 or Type II diabetes and gestational diabetes. So the term “diabetic” could refer to all three types.
Among non-diabetics, a policy of elective Cesarean for all suspected big babies over 8 lbs., 13 oz. means that a large number of pregnant people and babies would experience unnecessary surgeries. In order to prevent one permanent brachial plexus palsy in babies suspected to be over 8 lbs., 13 oz., 2,345 people would have unnecessary Cesareans at a cost of $4.9 million dollars per injury prevented (costs were estimated using year 1995 dollars).
With a policy of elective Cesareans for all suspected big babies over 9 lbs., 15 oz., even more pregnant people would have surgeries found to be unnecessary in retrospect, because ultrasounds are even less accurate in higher suspected weight ranges (Chauhan et al. 2005). In order to prevent one permanent brachial plexus palsy in babies suspected to be over 9 lbs., 15 oz., 3,695 people would need to undergo unnecessary Cesareans at a cost of $8.7 million per injury prevented.
Such policies would increase rates of known risks from Cesarean, like serious infections, blood clot disorders, postpartum bleeding (hemorrhage) requiring blood transfusions, and newborn breathing problems (see “” from ChildbirthConnection.org).
Among diabetics, the results were different—mostly because ultrasound is slightly more reliable at predicting big babies in pregnant people who are diabetic, and because shoulder dystocia is more common in this group as well. If pregnant diabeticswere offered an elective Cesarean for every baby that is suspected of weighing more than 8 lbs., 13 oz., it would take 489 unnecessary surgeries to prevent one case of permanent nerve damage, at a cost of $930,000 per injury avoided. If diabetics had elective Cesareans when their babies were suspected of being 9 lbs., 15 oz. or greater, it would take 443 unnecessary surgeries to prevent one case of permanent brachial plexus palsy, at a cost of $880,000 per injury avoided.
Please note: A cost-effectiveness analysis is only as good as its assumptions–the numbers that they use to plug into the analysis. For example, how did they determine how frequently shoulder dystocia occurs, the accuracy of ultrasounds, and how many permanent injuries occur? In the Rouse et al. (1996) paper, the authors did a very high-quality literature review to determine these factors. One drawback of this analysis is that the costs they reported did not include the cost of lawsuits.
Another important drawback is that this analysis is now over 20 years old.
Since the landmark Rouse et al. paper was published, two newer cost-effectiveness analyses have been published. However, both of these newer papers had major problems—one of them did not take into account the inaccuracy of ultrasound (Herbst, 2005), and the other researchers had a poor-quality systematic review—using numbers in their assumptions that overestimate the accuracy of ultrasound (Culligan et al. 2005). Because the researchers did not do a good job of making their assumptions, we cannot trust the results of their analyses, and so their results are not included in this Signature Article.
In summary, evidence does not support elective Cesareans for all suspected big babies, especially among non-diabetic pregnant people. There have been no randomized, controlled trials testing this intervention for big babies, and no high-quality research studies to see what happens when this intervention is used on a mass-scale in real life.
In fact, pregnant people without diabetes may be given one-sided information by their care providers if elective Cesarean is presented as a completely “safe” or “safer” option than vaginal birth for a suspected big baby. Although vaginal birth with a big baby carries risks, Cesarean surgery also carries potential harms for the birthing person, infant, and any children born in future pregnancies. It is important to have full information on both options in order to make a decision. To read more about the potential benefits and harms of Cesarean versus vaginal birth, you may want to read: “Vaginal or Cesarean Birth: What is at Stake for Women and Babies?” or the consumer booklet, “What every woman should know about Cesarean Section” from Childbirth Connection.
Guidelines
In 2016, the American Congress of Obstetricians and Gynecologists (ACOG) released an opinion stating that induction is not recommended for suspected big babies, because induction does not improve outcomes for birthing people or babies (recommendation based on “Level B evidence = limited or inconsistent evidence”). The 2016 practice bulletin was reaffirmed by ACOG in 2018. This recommendation is similar to their 2002 guidelines that were reaffirmed in 2008 and 2015, and eventually replaced by this new position statement published in 2016. In 2020, ACOG released another practice bulletin stating that more research needs to be done to determine whether the potential benefits of inducing for a suspected big baby to prevent shoulder dystocia before 39 weeks outweigh the risks of early induction (ACOG, 2020).
In 2008, the National Institutes for Health and Clinical Excellence (NICE) in the United Kingdom also An updated recommendation from NICE, released as a draft in May 2021, suggests that all pregnant people should be offered induction at 41 weeks, rather than allowing babies to grow for up to 42 weeks, to lower possible complications. This advice is not specific to suspected big babies and is based on expert opinion not clinical trials.
French practice guidelines from 2016 recommend induction for suspected big baby if the cervix is favorable at 39 weeks of pregnancy or more (Sentilhes et al. 2016). This recommendation is based on “professional consensus,” not research evidence.
In all their opinion statements since 2002, ACOG has stated that planned Cesarean to prevent shoulder dystocia may be considered for suspected big babies with estimated fetal weights more than 11 lbs. (5,000 grams) in birthing people without diabetes, and 9 lbs., 15 oz. (4,500 grams) in birthing people with diabetes.. They state the evidence is “Grade C,” meaning this recommendation is based on consensus and expert opinion only, not research evidence (ACOG 2002; ACOG 2013; ACOG 2016—Reaffirmed French guidelines on elective Cesarean for suspected big baby are consistent with the ACOG recommendation.
In summary, for those who are not diabetic:
- If a care provider predicts a big baby based on an ultrasound or physical exam, they will be right half the time and wrong half the time. Ultrasound weight results at the end of pregnancy can fall anywhere from 15% above or below the baby’s actual weight.
- About 7 to 15% of big babies have difficulty with the birth of their shoulders, which is known as shoulder dystocia. The chance of a permanent nerve injury due to shoulder dystocia happens in 1 out of every 555 babies who weigh between 8 lbs., 13 oz. and 9 lbs., 15 oz., and 1 out of every 175 babies who weigh 9 lbs., 15 oz. or greater. Regular training is important for health care providers to keep up their skills in managing shoulder dystocia, as this can help prevent injuries.
- If a care provider thinks that you are going to have a big baby, this thought is sometimes more harmful than the actual big baby itself. This is because the suspicion of a big baby leads many care providers to manage labor in a way that increases the risk of Cesarean and complications.
- Specifically, there is an increase in Cesareans for labor arrest or “stalled labor” when the care provider suspects a big baby, even if the baby’s actual weight is not big. So if a care provider thinks that you are going to have a big baby, it is especially important that they follow the standard definitions of slow labor (labor dystocia). Learn more here in our Evidence Based Birth® Signature Article on Cesareans for “Failure to Progress.”
- Although a policy of very early induction (37 to 38 weeks) can prevent some shoulder dystocia cases from occurring, researchers have not been able to show that induction decreases the risk of brachial plexus palsy, and very early induction may carry other risks to the birthing person or baby.
- A policy of elective Cesareans for big babies likely does more harm than good for most birthing people: It would take nearly 3,700 elective Cesareans to prevent one permanent case of brachial plexus palsy in babies who are suspected of weighing more than 9 lbs., 15 oz. B
For pregnant people with diabetes or gestational diabetes:
- Ultrasounds are slightly more accurate at predicting a big baby, because people with diabetes are at higher risk of having a big baby to begin with.
- Just like for birthing people without diabetes, it is also very important for care providers to follow the standard definitions of slow labor.
- Elective Cesareans may be more cost-effective in pregnant people who have Type I or Type II diabetes than they would be in those without diabetes.Based on expert opinion only, there may be some benefit to planned cesareans in diabetics with a suspected big baby estimated to weigh over 4500 grams (ACOG, 2020).
- Management of gestational diabetes (diet, exercise, or medication) lowers the chance of having a big baby and shoulder dystocia down to normal levels.


References:
- The American College of Obstetricians and Gynecologists, Committee on Practice Bulletins. (2020). ACOG Practice Bulletin: Macrosomia.
- org/clinical/clinical-guidance/practicebulletin/articles/2020/01/macrosomia
- Salihu, H., Dongarwar, D., King, L. et al. (2020). Phenotypes of fetal macrosomia and risk of stillbirth among term deliveries over the previous four decades. Birth: Issues in Perintal Care, 1-4, 8.
- onlinelibrary-wiley.com/doi/10.1111/birt.1247
- National Institute for Healthcare Excellence. (2021). Inducing Labour (update). [Epub ahead of print].
- https://www.nice.org.uk/news/article/nice-recommends-inducing-women-in-labour-earlier-in-new-draft-guidance
- Beta, J., Khan, N., Khalil, A., et al. (2019). Maternal and neonatal complications of fetal macrosomia: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. [Epub ahead of print]. Click here.
- Jolly, M. C., Sebire, N. J., Harris, J. P., et al. (2003). Risk factors for macrosomia and its clinical consequences: a study of 350,311 pregnancies. European Journal of Obstetrics & Gynecology and Reproductive Biology, 111, 9-14. Click here.
- Araujo Júnior, E., Peixoto, A. B., Perez Zamarian, A. C., et al. (2017). Best Practice & Research Clinical Obstetrics & Gynaecology, 38, 83-96. Click here.
- Tuuli, M. G., Kapalka, K., Macones, G. A., et al. (2016). Three-Versus Two-Dimensional Sonographic Biometry for Predicting Birth Weight and Macrosomia in Diabetic Pregnancies. J Ultrasound Med, 35(9), 1925-30. Click here.
- Rui-Xue, D., Xiu-Jie, H., and Chuan-Lai, H. (2019). The Association between Advanced Maternal Age and Macrosomia: A Meta-Analysis. Childhood Obesity, 15, 3. Click here.
- Dude, A. M., Grobman, W. A. and Yee, L. M. (2018). Association between Sonographic Estimated Fetal Weight and the Risk of Cesarean Delivery among Nulliparous Women with Diabetes in Pregnancy. Am J Perinatol., 35(13):1297-1302. Click here.
- Dude, A. M., Davis, B., Delaney, K., et al. (2019). Sonographic Estimated Fetal Weight and Cesarean Delivery among Nulliparous Women with Obesity. AJP reports, 9(2), e127–e132. Click here. Free full text
- Fang, F., Zhang, Q. Y., Zhang, J., et al. (2019). Risk factors for recurrent macrosomia and child outcomes. World J. Pediatr, 15(3), 289-296. Click here.
- Kadji, C., Cannie, M. M., Carlin, A., et al. (2019). Protocol for the prospective observational clinical study: estimation of fetal weight by MRI to PREdict neonatal MACROsomia (PREMACRO study) and small-for-gestational age neonates. BMJ Open, 9, e027160. Click here. Free full text!
- Preyer, O., Husslein, H., Concin, N., et al. (2019). Fetal weight estimation at term – ultrasound versus clinical examination with Leopold’s manoeuvres: a prospective blinded observational study. BMC Pregnancy and Childbirth, 19, 122. Click here. Free full text!
- Sancetta, R., Khanzada, H. and Leante, R. (2019). Shoulder Shrug Maneuver to Facilitate Delivery During Shoulder Dystocia. Obstetrics & Gynecology, 133(6), 1178-1181. Click here. Free full text!
- Weiss, C., Oppelt, P., and Mayer, R. B. (2018). Disadvantages of a weight estimation formula for macrosomic fetuses: the Hart formula from a clinical perspective. Archives of gynecology and obstetrics, 298(6), 1101–1106. Click here. Free full text!
- Davenport, M. H., Meah, V. L., Ruchat, S.-M., et al. (2018). Impact of prenatal exercise on neonatal and childhood outcomes: a systematic review and meta-analysis. Br J Sports Med, 52, 1386–1396. Click here. Free full text!
- Kamana K. C., Shakya S. and Zhang, H. (2015). Gestational Diabetes Mellitus and Macrosomia: A Literature Review. Ann Nutr Metab, 66(suppl 2), 14-20. Click here. Free full text!
- Wood, S. and Tang, S. (2018): Stillbirth and large for gestational age at birth, The Journal of Maternal-Fetal & Neonatal Medicine. [Epub ahead of print]. Click here.
- Milner, J. and Arezina, J. (2018). The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: A systematic review. Ultrasound, 26(1), 32–41. Click here.
- Sentilhes, L., Senat, M. V., Boulogne, A., et al. (2016). Shoulder dystocia: guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF). Eur J Obstet Gynecol Reprod Biol, 203, 156-61. Click here.
Resources:
- To read our Signature Article on Diagnosing Gestational Diabetes, click here
- To read our Signature Article on Inducing Labor for Gestational Diabetes, click here
- For an interesting article about women’s experiences being told they have suspected big babies, click here.
- For a video demonstrating why you should not pull on a baby’s head during shoulder dystocia, click here.
- To follow the Erb’s Palsy Facebook page (for Brachial Plexus Palsy) click here, and to join their public support group (“Oliver’s Friends”), click here.
Acknowledgments:
We would like to extend our gratitude to our expert clinician reviewers who have reviewed past versions of this article: Emiliano Chavira, MD, FACOG, FSMSM and Angela Reidner, MSN, CNM, who reviewed the article in 2016, and Amy Romano, MSN, CNM, co-author of Optimal Care in Childbirth: The Case for a Physiologic Approach, Robert Modugno, MD, MBA, FACOG, and Shannon J. Voogt, MD, Board-Certified in Family Medicine, for their feedback on the original version of this article published in 2012.
I would also like to thank Arianna Taboada, MPH; Mandi Garvey, MPH, EMT, CD(DONA); Fiona Cunningham, M.Ed., C.C.C. for their thoughtful feedback on the original version of the article, as well as Cristen Pascucci and Sharon Muza CD(DONA), BDT(DONA), LCCE, FACCE for their medical editing assistance of past versions.
Birth Professionals:
Join others who also want to help bring evidence-based care to their local community.
Also, gain complimentary access to a printable library of our Signature Articles, 20+ hours of CE courses, a private community, and more.
Buy EBB Inspirational T-shirts, Due Date Buttons & Birth Affirmation Cards




Stay empowered, read more :
EBB 308 – The Intersection of Environmental Justice and Midwifery Care with Dr. Tanya Khemet Taiwo
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | YouTube Curious which toxins should be most avoided for people of reproductive age and their children? In this episode, Dr. Tanya Khemet Taiwo, LM, CPM, MPH, PhD unravels the...
EBB 307 – Unexplained Infertility, Endometriosis, and a Birth Center Birth Story with Ellora La Shier, EBB Childbirth Class Graduate
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | GoogleIn this episode, Ellora La Shier, graduate of the EBB Childbirth Class, shares about her struggle with six years of unexplained infertility and how it impacted her...
EBB 306 – Culturally Sensitive Doula Training with Divya Deswal and Neha Misra of the Doula Collective in India
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | Google As we wrap up the celebration of World Doula Week, explore the transformative journey of pregnancy and childbirth in the Indian context with Divya Deswal and Neha Misra,...



