
PhD, RN
Evidence on: Eating and Drinking During Labor
Updated by Rebecca Dekker PhD, RN on May 3, 2022. Originally published in 2013. © Evidence Based Birth®, All Rights Reserved. Please read our Disclaimer and Terms of Use. For a printer-friendly PDF, become a Professional Member to access our complete library.
In many hospitals, patients are told not to eat or drink during labor. The medical term for this is “NPO,” which comes from the Latin nil per os, meaning nothing by mouth. In a survey of people who gave birth in U.S. hospitals, 60% reported not drinking during labor, and 80% said that they did not eat (Declercq et al. 2014). However, when people are free to eat and drink as desired during labor, as is typical in U.S. freestanding birth centers, very few of them (5%) choose to not eat or drink (Rooks et al. 1989).
What are the body’s nutritional needs during labor?
The uterus is mostly made of muscle tissue. Muscles use fuel as they work and require enough nutrition to meet their energy needs.
Very little research has been published on nutritional needs during labor, but research in sports nutrition has found that ingesting carbohydrates during exercise improves performance and protects against fatigue and ketosis (Rodriguez et al. 2009). Ketosis means that there are raised levels of ketones that can be measured in blood and urine. During times of starvation or carbohydrate restriction, the body burns fat for energy, resulting in the release of ketones. It’s not clear whether ketosis during labor is normal and harmless or if it requires an intervention like IV fluids or food and drink (Toohill et al. 2008).
In a news release about research on eating and drinking in labor, one researcher in the field said laboring people’s energy/calorie needs are similar to those of marathon runners. Online advice for marathon runners is to aim for roughly 3 grams of carbohydrates per kilogram of your body weight before the race. So, larger people and smaller people might have different carbohydrate requirements to meet energy needs during labor.
So far, we do not have evidence on specific foods or drinks to recommend for consumption during labor. Some foods specifically mentioned in studies (Ciardulli et al. 2017; Karimi et al. 2020; Huang et al. 2020) include:
-
- Oral carbohydrate-based fluids
- Date fruit or date syrup
- Low fat yogurt
- Bread, biscuits
- Vegetables
- Fruits
- Soup
- Fruit juices
- Cereal and milk
- Toast with butter/jam
- Low fat cheese
- Chocolate
- Boiled eggs
We commonly hear doulas recommend consuming honey sticks and coconut water during labor. In addition, doulas on our Team here at Evidence Based Birth® suggest laboring people consider eating foods that are affordable and culturally grounded for you. Some examples from Team EBB include:
-
- Roasted okra
- Tortillas with a honey or tahini spread
- Tostones
- Fried plantain (maduros) bites in light oil
In the U.S., some “cultural foods” may be perceived as unhealthy for laboring people due to their foods being perceived as “strong-smelling,” “spicy,” or “heavy/greasy.” Most dieticians, nurses, doctors, and midwives in the U.S. are white (U.S. DHHS 2017; Serbin & Donnelly, 2016), and we’ve seen health care workers make disgusted facial expressions or remarks when they see a patient eating “ethnic” food. We have also witnessed labor and delivery staff make judgmental and classist statements about people who would like to eat fast food during labor.
Someday we may have evidence on the best foods to fuel labor. But regardless of any research that may come out, here at EBB we believe that food choices should ultimately be left up to the birthing person’s preferences and desires. We urge nurses, doctors, and midwives to address their own implicit biases about food and question their assumptions about what makes something healthy or unhealthy to eat during pregnancy or labor. As Maya Feller, MS, RD, CDN says, “Looking down on another culture’s food demonizes one of the major pillars that makes that culture who they are.”
What if you have pregestational Diabetes or gestational Diabetes?
We did not find any evidence or guidelines on eating or drinking during labor for people with pregestational (pre-existing) diabetes or gestational diabetes. However, there are some guidelines on glycemic (i.e. blood sugar) control during childbirth.
Even if glucose levels are well controlled throughout pregnancy, glycemic control over the 18 hours or so before birth has a significant impact on the newborn (AACE, 2022). When the birthing person has hyperglycemia (high blood sugar) during labor, the baby compensates by secreting more of the hormone insulin, which can result in fetal hyperinsulinemia. Then after the umbilical cord is clamped, the source of incoming glucose is cut off and the newborn can experience hypoglycemia (low blood sugar).
Labor has a glucose lowering effect, just like exercise (AACE, 2022). For this reason, people with gestational diabetes who usually require insulin should stop the insulin at the start of labor. There is no mention of eating/drinking, but the AACE recommends, “sufficient glucose should be infused to keep the woman from becoming ketotic from the pronged period of starvation.”
ACOG has a practice bulletin on pregestational diabetes with a section on glucose management during labor (ACOG, 2018). They recommend:
-
- Once active labor begins or glucose levels decrease to less than 70 mg/dL, the infusion is changed from normal saline to 5% dextrose and given at a rate of 100–150 cc/h (2.5 mg/kg/min) to achieve a glucose level of about 100 mg/dL.
- Glucose levels are checked hourly using a bedside meter allowing for adjustment in the insulin or glucose infusion rate.
- Regular (short-acting) insulin is given by IV infusion at a rate of 25 units/h if glucose levels exceed 100 mg/dL.
The UpToDate article on “Intrapartum and postpartum glycemic control” for people with pregestational diabetes or gestational diabetes seems to assume that people will not be allowed to have oral intake during labor. They recommend eating 50% of normal caloric intake during the cervical ripening period of a medical labor induction (as it can take 12-24 hours to complete cervical ripening before labor contractions are started).
Here at EBB, in our communications with pregnant people with pregestational or gestational diabetes, many of them have voiced their struggles when staff forbid them from using nutrition to manage blood sugar during labor. Here are some examples of situations we have heard about:
-
- A pregnant person with gestational diabetes has managed their blood sugar with diet alone. The medical team admitting the patient tells them that they are NPO. The patient is scared and upset that they are not supported in managing their blood sugar with nutrition during labor.
- Someone with pregestational diabetes has an insulin pump and pays strict attention to their glucose levels, insulin requirements, and nutrition needs. This person, who has successfully managed their own glucose years for many years, is told they must be “NPO” for labor, and that their glucose levels will now be managed by the health care team. The patient worries that the medical staff, who are not as familiar with the intricacies of their body, could unintentionally cause episodes of hypoglycemia or hyperglycemia.
- A person with gestational diabetes wants to keep up their fluid intake during birth, but they are put on a “clear liquids only” diet. The only liquids that are brought to their room are sugary fluids like jello, popsicles, and juice. Their blood sugar spikes, and they are given insulin (which they’ve never needed to use before). The insulin dose is too high, and their blood sugar crashes, which leads them to need a glucose infusion, and it takes hours to stabilize their glucose levels.
Because of NPO and clear liquids only policies in hospitals, some of the diabetic parents we talk to must bring their own food and drinks with them to the hospital.
Why do hospitals use “NPO in labor” policies, anyways?
The “Nothing by Mouth” policy during labor began in the 1940s, when several practices were quite common:
-
- Twilight Sleep, an intravenous injection of morphine and scopolamine, was used for patients giving birth in hospitals. This medication combination caused sedation (ranging from mild to deep) and no memory of the birth afterwards. However, pain relief was only available for white patients; Black patients were typically excluded from receiving Twilight Sleep or any other form of medication pain relief.
- Most white patients were also given inhaled anesthetics (gas, also known as general anesthesia) to make them unconscious for the traditional episiotomy + forceps-assisted vaginal delivery of the baby. Back then, the gases of choice were ether or chloroform, and they were given in inexact (imprecise) amounts. As you can imagine, anesthesia in the 1940s was more dangerous, and aspiration was more common than it is today.
Aspiration is when a person vomits stomach contents into their mouth while under anesthesia. If the contents of the stomach are aspirated back down the airway—going down the “wrong tube”— then this can lead to infection and breathing problems, called aspiration pneumonitis. Due to the possible risk of aspiration, general surgery patients are often asked to fast for at least eight hours before scheduled procedures (however, as we will discuss later, some fasting policies for general surgery are being rejected due to new evidence).
In the 1940s, when aspiration was recognized as a major problem during birth, anesthesiologists were using very primitive tools to keep a person’s airway open when under general anesthesia, and some doctors didn’t use any airway tools at all.
New versions of a tool called a laryngoscope were developed in the late 1940s, allowing doctors to view a patient’s vocal cords so that they could place a tube in the trachea (intubation) and keep an open and protected airway during general anesthesia (Robinson & Toledo, 2012). The design, technique, and popularity of laryngoscopes and intubation continued to improve over the second half of the 20th century.
Before these advances, in 1946, Dr. Curtis Mendelson published the landmark study responsible for “Nothing by Mouth” policies. He described how giving general anesthesia during birth could lead to the inhalation of stomach contents, which in rare cases could lead to severe lung disease or death. The pathology of this illness, named “Mendelson’s syndrome,” was replicated in animal studies (Mendelson, 1946).
Content note for next paragraph: Pregnancy-related deaths due to anesthesia
When Dr. Mendelson looked at 44,016 patients who gave birth from 1932 to 1945, he found that aspiration occurred in 66 of them (0.15% or 1 in 667). All the people who experienced aspiration had a mixture of gas, ether, and oxygen given to them through a mask during the delivery. It is not clear if any of them had airway protection. General anesthesia wasn’t limited to Cesarean deliveries; it was also used to control the patient during vaginal births. More than half of the people in the study had a longer anesthesia time and greater anesthesia depth than usual. Most of the aspirations were from liquids, and only a few were from solids. There were two deaths in the study; both patients had general anesthesia without airway protection, aspirated solid food, and died of suffocation on the delivery table.
Mendelson concluded that aspirations are preventable and recommended using IV fluids instead of oral fluids. He also recommended switching to local anesthesia when possible, instead of general anesthesia. His advice caught on, and “Nothing by Mouth” became the norm in hospitals across the U.S. and even around the world. The NPO practice has persisted, becoming a part of hospital culture, even though the modern population is nothing like the people who gave birth back in Dr. Mendelson’s time, who were exposed to general anesthesia all the time, and without airway protection.
To learn more about Mendelson’s syndrome, you can read this public article.
What is the risk of death from aspiration?
Content note for this section: pregnancy-related deaths due to anesthesia.
Let’s jump ahead to 1997, when researchers conducted the first large U.S. study to look at pregnancy- related deaths due to anesthesia between the years 1979 to 1990. General anesthesia was used in 41% of the sample in the earlier years, and 16% of the sample in the later years. The risk of death because of aspiration during Cesarean was 1 death for every 1.4 million births (Hawkins et al. 1997).
A follow-up study looked at anesthesia and pregnancy-related deaths in the U.S. between 1991 and 2002 (Hawkins et al. 2011). During this time, general anesthesia was used in approximately 14% of births. They found that anesthesia-related deaths fell 60% over time. The authors calculated that there were 6.5 deaths per million uses of general anesthetics from the later years in the sample (1997-2002). The number of these deaths directly caused by aspiration was not studied because it was too difficult to distinguish them from the other deaths related to airway problems, such as intubation problems, inadequate ventilation, or respiratory failure (Personal correspondence, Hawkins, 2016).
Similarly, a study in Michigan between 1985 and 2003 reported eight anesthesia-related deaths among pregnant people. Five of the eight deaths involved general anesthesia; none of the participants in this study died from aspiration (Mhyre et al. 2007).
Some people may argue that the reason there are fewer deaths from aspiration today is because people are not allowed to eat or drink during labor. However, in the United Kingdom, clinical guidelines were updated in 2007 to recommend that drinks and a light meal be offered to low-risk people in labor. So, it may be helpful to look at aspiration deaths in the United Kingdom since 2007, after they began to encourage eating and drinking during labor.
The United Kingdom reviews every pregnancy-related death in regular “Confidential Enquiries into Maternal Deaths Reports.” Between 2000 and 2008 (spanning three reports), one person died from aspiration out of more than six million births (Cantwell et al. 2011).
The death occurred between 2006 and 2008, but it’s not clear whether this was before or after the change in guidelines.
The person in this case had a known placenta previa and was hospitalized for monitoring, but was not in labor. After consuming a full meal in the hospital, the patient began bleeding due to the previa and had an emergency Cesarean with general anesthesia. Vomiting occurred while the tube was being removed in the recovery room, and the patient died a few days later from the resulting aspiration pneumonitis.
The report recommends that when general anesthesia is administered in a suspected full stomach situation, the person should ideally be fully awake and able to protect their airway when it comes time for the tube to be removed (a procedure known as extubation). Attempts to reduce stomach contents with a tube inserted into the stomach through the mouth (orogastric tube) should have also taken place, but did not.
A second maternal death from anesthesia-related aspiration occurred almost 10 years later, between 2013 and 2015 (Knight et al. 2017). In this case, a pregnant woman with small bowel obstruction (blockage in the small intestine) aspirated during a combined Cesarean and general surgical procedure. The health care workers did not place a nasogastric tube in the patient with the general anesthesia. The authors say that this tube should have been placed to empty the stomach, as this is standard practice during surgery with conditions such as bowel obstruction.
We have heard many doctors say that everyone going into labor is assumed to be at risk of aspiration (because it is not possible to predict who will end up needing a Cesarean surgery under general anesthesia), so everyone should be NPO during labor. However, the studies above show that aspiration death is extremely rare during childbirth. The few published deaths that we found were completely preventable—standard airway protection was not provided. Overall, a small percentage of Cesareans require general anesthesia today, and when they do, failed airway management is rare.
How often does illness from aspiration occur?
Content note for this section: discussion of cardiac arrest.
In 1989, researchers looked at 11,814 low-risk people who gave birth in 84 freestanding U.S. birth centers from 1985 to 1987 (Rooks et al. 1989). There were no aspirations, even though 95% of the study participants drank or ate while in labor. Only 4.4% of those planning a home birth transferred to the hospital for a Cesarean; it’s not clear how many of the Cesareans were performed under general anesthesia. This sample population was at especially low-risk of aspiration because of the low rate of surgical births.
In 2014, researchers looked at 57 million hospital births in the U.S. between 1998 and 2011 to better understand cardiac arrest in people giving birth (Mhyre et al. 2014). Cardiac arrest is an emergency that happens when the heart suddenly stops beating. The researchers found that cardiac arrest happened in 1 in 12,000 birthing people and that aspiration pneumonitis possibly contributed to 346 out of 4,843 (7%) of these cardiac arrests. This means that about 6 cardiac arrests per million births may have been related to aspiration.
A few notes about this study:
-
- The researchers relied on diagnosis codes and did not have access to the actual medical records. This means that they cannot tell which came first—the aspiration, or the cardiac arrest. Some of the birthing people may have had cardiac arrest due to another cause, and then experienced aspiration as a complication of the arrest.
- It’s also not possible to tell from this study how many of the 346 aspirations occurred in high-risk births. Preeclampsia/eclampsia, for example, increases the odds of cardiac arrest by 7 times.
- Most (83%) of those who experienced both cardiac arrest and aspiration pneumonitis survived to hospital discharge.
North America’s Society for Obstetric Anesthesia and Perinatology developed a registry of obstetric anesthesia complications between 2004 and 2009 (D’Angelo et al. 2014). Thirty U.S. hospitals provided information on more than 307,000 people giving birth. Most of the birthing people (257,000) had regional (epidural, spinal or combined spinal-epidural) or general anesthesia. General anesthesia accounted for 5.6% of Cesareans in this study. Out of 5,000 pregnant people who received general anesthesia, there were zero cases of aspiration. We don’t know how many ate or drank during labor.
The Royal College of Anaesthetists and the Difficult Airway Society conducted a study to estimate how often major airway events (also called “near deaths”) occurred during general anesthesia in the U.K. (Cook et al. 2011). Out of approximately 720,000 births that took place during 2008-2009, only one case of aspiration was documented. And the aspiration wasn’t considered the primary cause of the person’s airway problems. Instead, the main complication was because they had difficulty placing a tube in this person’s airway. We don’t know what the oral intake was during labor, only that the individual transferred from a midwifery unit for a long pushing stage and had a Cesarean with regional anesthesia…but then needed general anesthesia during the surgery. The birth resulted in a live infant and the birthing parent made a full recovery within a week.
More recently, a 2-year national descriptive study from the U.K. examined aspiration during pregnancy and the immediate postpartum period between 2013 and 2015 (Knight et al. 2016). They found nine confirmed cases of aspiration out of nearly 1.5 million pregnancies, giving an estimated rate of only 6 aspiration events per million pregnancies. Seven of the cases occurred with general anesthesia, representing 2.2 cases per every 10,000 uses of general anesthetics. The other instances of aspiration occurred when pregnant people were semi-conscious for other reasons. One person died from aspiration during this period (described earlier).
The authors write that aspiration in pregnancy and immediately postpartum in the U.K. is extremely rare: “Reassuringly, there does not appear to be a substantial number of cases associated with oral intake in labor following the change in policy [to no longer restrict oral intake among low-risk people in labor.]”
What impact do NPO policies have on birth today?
In a Cochrane review, researchers combined evidence from five trials involving a total of 3,103 participants who were randomly assigned to eat/drink or not during labor (Singata et al. 2013). Everyone was in active labor and at low risk of needing a Cesarean. A few of the trials reached opposite conclusions on outcomes like Cesareans, vomiting, and labor duration. Unfortunately, none of the researchers looked at satisfaction with childbirth. They concluded that there is no proven harm or benefit in restricting low-risk people from consuming food and drink during labor.
Table 1 shows details about the five randomized trials in the Cochrane review.
Table 1:Singata et al. 2013 Meta-Analysis on Eating or Drinking During Labor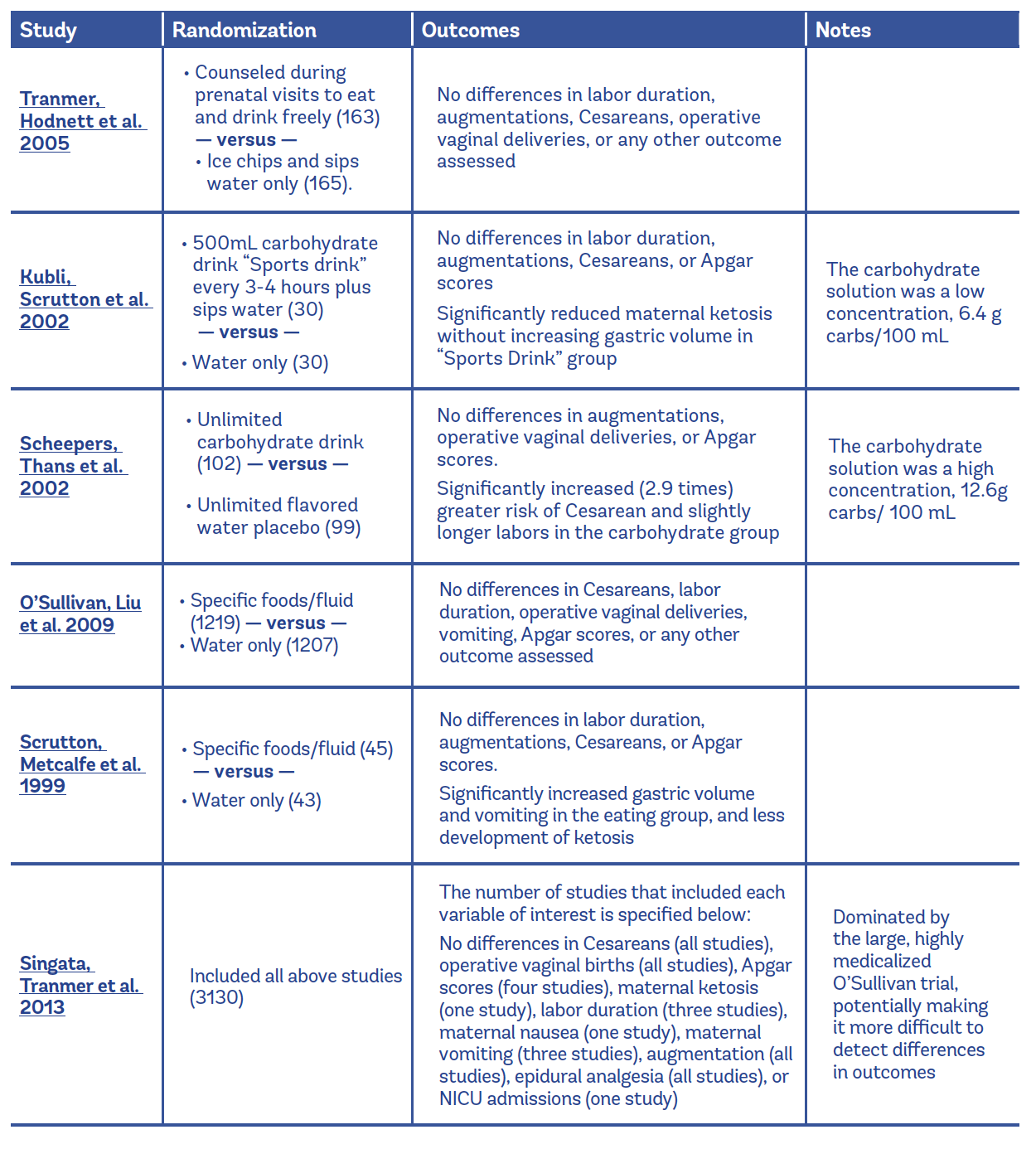
In 2017, another review described the benefits and harms of food and drink during labor (Ciardulli et al. 2017). The researchers included all five studies from the Cochrane review and added five more, for a total of 3,982 participants. The authors found that less restrictive eating and drinking policies led to shorter labors by about 16 minutes. There were no differences in any other health outcomes.
Only one of the trials considered satisfaction and found that more of the eating group participants reported satisfaction with their nourishment during labor compared to those given sips of water only (97% versus 55%).
Table 2 shows details about the five additional randomized trials included in the Ciardulli et al. review.
Table 2: Ciardulli et al. 2017 Meta-Analysis on Eating or Drinking During Labor
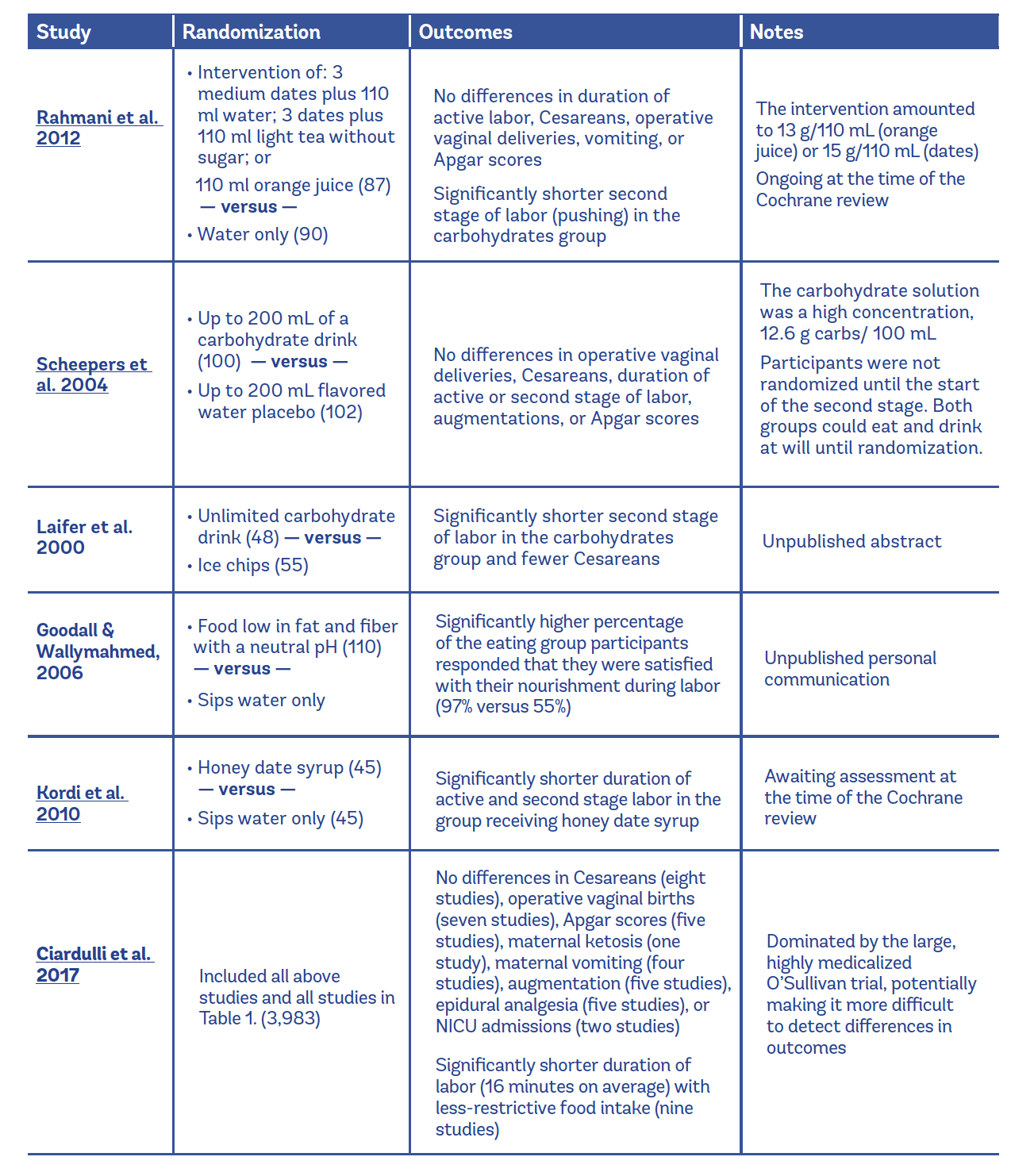
There were no cases of aspiration in any of the trials; however, the study sizes were not large enough to determine how often this rare outcome truly occurs.
The authors of the Cochrane review note that most laboring people seem to naturally limit their intake as labor gets stronger. They concluded that if you’re low risk, you should be able to choose whether you would like to eat and drink during labor (Singata et al. 2013). No trial has examined eating during labor in people who are at higher risk of needing Cesareans with general anesthesia.
Interestingly, in a 2016 position statement update, the American Association of Anesthesiologists reviewed much of the same evidence and decided that because there isn’t evidence of harm or benefit, hospitals should limit solid food during labor. Patient satisfaction was not factored into their opinion.
We found four studies that surveyed birthing people on their perceptions of food and drink restrictions during labor.
-
- The first study, conducted in Iran, interviewed 600 people, and found a relationship between reported pain levels and environmental sources of stress, in which laboring people with higher levels of stress had higher levels of pain (Manizheh & Leila, 2009). One of the greatest reported sources of stress was “restricted fluid ” About half of first-time birthing people and 78% of people who had given birth before mentioned this as a stressor.
- In a second study, also from Iran, researchers conducted in-depth interviews with 24 low- risk people after they had given birth, but before leaving the hospital (Iravani et 2015). The participants were in three different hospitals, demographically diverse, and all had healthy infants. The interview responses were grouped into common themes and coded for data analysis. One of the reoccurring responses was disappointment about restrictions on eating and drinking during labor. Participants commented that they “felt out of energy,” “had no more strength,” and “felt hungry from going so long without eating.”
-
- British Columbia Women’s Hospital in Canada conducted a recent survey of laboring people’s oral intake preferences (Liang et al. 2021). The hospital was anticipating a change from their current “clear fluids only” policy to a less restrictive policy. They wanted to gather information from people giving birth under the current restrictive policy. The survey asked people during labor or after the birth (postpartum) whether they would have liked to eat during labor and to rank their order of preference for several options. They obtained 315 responses, 165 people who took the survey during labor, and 150 who completed the survey postpartum.
The researchers found that:
- British Columbia Women’s Hospital in Canada conducted a recent survey of laboring people’s oral intake preferences (Liang et al. 2021). The hospital was anticipating a change from their current “clear fluids only” policy to a less restrictive policy. They wanted to gather information from people giving birth under the current restrictive policy. The survey asked people during labor or after the birth (postpartum) whether they would have liked to eat during labor and to rank their order of preference for several options. They obtained 315 responses, 165 people who took the survey during labor, and 150 who completed the survey postpartum.
-
-
- Most people (74%) who completed the survey during labor reported a desire to eat.
- Fewer people (53%) recalled a desire to eat during labor when they were interviewed postpartum. The researchers think this difference could relate to when the survey was completed relative to the respondent’s last meal (whether someone completed the survey with an empty or full stomach).
- People who had epidurals were significantly more likely to report a desire to eat during labor.
- The overall preferred food options were fruits and vegetables, and water was the preferred option for fluids.
-
-
- Another survey study from Australia included 149 postpartum parents who had given birth at the same hospital (McDermott et al. 2022). There are currently no Australian recommendations regarding eating and drinking during labor. The aim of this survey was to help inform future recommendations, considering laboring people’s preferences experiences and Everyone 18+ years of age who had given birth within the last seven days and had experienced active labor was invited to participate in the study.
The researchers found that:
-
-
- Most people (83%) strongly agreed or agreed that they felt like drinking during labor, and a third used carbohydrate-containing drinks.
- Fewer people (30%) strongly agreed or agreed that they felt like eating during labor.
- More than half (61%) of respondents reported experiencing nausea during labor and 32% reported vomiting in labor.
- When asked about advice they had received from a health care professional, one in four respondents did not receive any advice about eating and drinking during labor Those who did receive advice reported conflicting guidance from many different sources.
- Most people spent about five and a half hours in active labor, but the longest duration was over 20 hours (a long time to abstain from eating or drinking during physical exertion).
-
We found two studies examining national practices around oral intake during labor.
- In China, a study of more than 1,200 hospitals found many differences between hospitals in their oral intake policies during labor (Huang et al. 2020).
- 77% of hospitals allowed pregnant people to bring easily digestible food.
- 67% allowed pregnant people to eat and drink as desired during labor.
- 9% did not allow solid food during labor.
- 3% did not allow water or other drinks.
- Interestingly, more than half of the hospitals (61%) specifically adopted the measure of eating chocolate during labor and 9% mentioned boiled eggs. Both of these foods are thought to digest and absorb quickly.
- Around 8% of hospitals provided a light meal for laboring people.
- According to the authors, more hospitals in China should provide a suitable diet for labor.
- An online survey in the Czech Republic questioned 52 care providers, mostly midwives and doulas, about their perceptions of the level of respectful or non-respectful care given to laboring people (Begley et al. 2018).
- The respondents worked in one or more of 51 hospitals or with home births
- One of the questions participants were asked was whether people in normal labor, with no risk factors, were permitted to drink fluids and eat a light meal in labor.
- The respondents all reported that low-risk people laboring at home were permitted to drink fluids and eat a light diet.
- Most respondents (95%) said that low-risk people laboring in the hospital were allowed to drink fluids, and fewer (76%) said they could eat a light diet.
- The authors suspect that the “unjustified” restriction of fluids and food in labor may be contributing to the unnecessary use of IV fluids, a practice which half of respondents said occurred ‘always’ or ‘frequently’ in the hospital.
Ultimately, people have the human right to decide if they would like to eat or drink during labor, or not. Hospital policy is not binding on patients, including birthing people, and hospitals do not have the legal authority to prevent a laboring person from eating and drinking if they so choose.
Findings presented at the Anesthesiology Annual Meeting
In 2015, several researchers at the annual meeting of anesthesiologists in the U.S. reported their research findings that most healthy people would benefit from a light meal in labor (Harty et al. 2015). To read a news release about this study, click here.
The researchers combined 385 research studies of hospital births published in 1990 or later. They also reviewed the American Society of Anesthesiology’s (ASA) Closed Claims Project database. In all, they found only one case of aspiration in the U.S. between 2005 and 2013, in a woman who was plus size and had pre-eclampsia. They concluded that fasting is not necessary in low-risk laboring people. In fact, fasting can lead to ketosis, making stomach juices more dangerously acidic if there were an aspiration.
The reviewers mentioned a few circumstances that can increase risk of aspiration – eclampsia, pre- eclampsia, having a body mass index (BMI) of 30 and above, and the use of intravenous (IV) opioids (such as morphine) to manage labor pain (which may further delay stomach emptying). They ended by saying that more research focusing on high-risk birth is needed, but people with these risk factors could possibly benefit from fasting during labor.
In an interview we did with the authors of this study, they said that the anesthesiology profession has made great progress since the 1940s. Even though Cesarean rates have risen as high as 32% of all U.S. deliveries, widely increased use of regional anesthesia during surgery, such as a spinal or an epidural, has resulted in far fewer anesthesia-related pregnancy deaths. When a general anesthetic is used, doctors now use new strategies to reduce the volume of stomach contents, make stomach juices less acidic (by administering medications), and keep the person’s airway safe. These advances were not available back in Dr. Mendelson’s time (Personal communication, M. Bautista, 2015).
Recall that the large Hawkins et al. 1997 study (of around 45 million births) looked at birth and death certificates and found the risk of aspiration death during delivery to be 0.7 per million people. That estimate is from a sample in the 1980s, before general anesthetic use decreased from 41% of all Cesareans to less than 6% now (nearly all involving emergent situations) (D’Angelo et al. 2014), and before pregnancy-related deaths fell an additional 60% (Hawkins et al. 2011). SSo, the risk of aspiration during surgery under general anesthesia is likely even lower today than in 1997, the last time we have exact numbers published about aspiration death in the U.S. population. As it says in a recent Anesthesiology editorial, “The actual incidence of the complication is so low, we cannot accurately describe it” (Palmer and Jiang, 2022).
The researchers who presented at the 2015 ASA meeting concluded that “Nothing by Mouth” is an outdated restriction that should not be applied to low-risk people giving birth today. Their findings were echoed in a 2016 opinion paper published by Sperling et al. in the American Journal of Obstetrics and Gynecology.
Some people have (inaccurately) taken this publication to mean that the American Society of Anesthesiologists supports eating during labor, since the publication was presented at the ASA Annual Meeting. However, the ASA continues to deny that patients should have the right to eat during labor, as we’ll discuss in a few sections down.
How does labor (and epidural use) affect stomach emptying?
The main reason that some hospitals have a “Nothing by Mouth” policy is to ensure that laboring people have an empty stomach should they need emergency surgery with general anesthesia. But is this effective?
We also hear that many hospitals who have lessened their restrictions still ban food for patients with epidurals during labor. Is there evidence to back up these eating bans?
We found three small studies on these topics:
-
- In 1992, researchers used ultrasound imaging to look at the stomach contents of 39 healthy, full- term pregnant people in active labor after they had received epidurals (Carp et al. 1992):
- The participants told the researchers (but not the person giving the ultrasound exam) when they had last eaten.
- The ultrasound found solid food in nearly two-thirds of the participant’s stomachs.
- Of the 25 who reported not eating for 8-24 hours, 16 still had solid food in their stomachs at the time of the ultrasound.
- Importantly, the presence of solid food in the stomach was not related to how long a person had gone without eating.
- In 2014, researchers did stomach ultrasound measurements in 60 laboring people with epidurals to track the changes in their stomach contents during labor (Bataille et al. 2014):
- In early labor, half of the participants had stomach contents considered likely to be a risk for aspiration, even though most of them had been without liquids for more than five hours and solids for more than 13 hours.
- However, by the pushing stage, nearly 90% of the participants in this study were no longer at risk for aspiration.
- The researchers concluded that neither the length of fasting nor the presence of stomach contents at the start of labor were good indicators of aspiration risk further along in labor.
- Although labor slows down stomach emptying, the stomach continues to empty during labor.
- A small prospective study from France offers new evidence on the effect of epidural use on stomach emptying after a solid meal (Bouvet et al. 2022):
- The researchers enrolled and tested a total of 40 healthy subjects: 10 nonpregnant females, 10 people who were pregnant at term, 10 laboring people with epidurals, and 10 laboring people without epidurals.
- Everyone had an empty stomach (confirmed with ultrasound) and then ate a light meal of less than five ounces of yogurt within five minutes.
- More ultrasound measurements of the stomach were taken at 15, 60, 90, and 120 minutes.
- They found that stomach emptying of a light meal was delayed in laboring people compared to nonpregnant females and pregnant people not in labor.
- The main finding of this study, however, was that epidural use did not worsen stomach emptying but rather helped with stomach emptying.
- Two hours after eating the light meal, only 3 of the 10 people laboring with epidurals still had solid food in their stomach, compared to 9 out of 10 of the laboring people without epidurals.
- Click here to view a free infographic of this study’s findings.
- In 1992, researchers used ultrasound imaging to look at the stomach contents of 39 healthy, full- term pregnant people in active labor after they had received epidurals (Carp et al. 1992):
So, what can we make of this research about stomach emptying and epidural status?
Importantly, stomach emptying slows down once labor starts, so fasting for 8, 12, or even 24 hours after contractions begin may not guarantee an empty stomach at the time of birth. This means that withholding food is not effective in creating an empty stomach situation.
The authors of the third study (Bouvet et al. 2022) think that the significantly lower pain scores seen in the epidural group may have improved stomach emptying. The authors concluded that epidural use “should be taken into consideration when allowing women in labor to consume a light meal.” In other words, they see people with epidurals as good candidates for eating during labor (in contrast to many doctors, who withhold food from laboring people with epidurals).
However, we would caution against saying people should or shouldn’t eat in labor based on whether they have an epidural. These studies are small, and there are serious ethical concerns with restricting food based on whether people choose to have an epidural for pain management or not. People who have an epidural may have longer labors and wish to eat to sustain their energy. And people who do not have an epidural (especially those who prefer to avoid unnecessary interventions) may also feel very strongly about their right to eat during labor.
Professional guidelines
In the guidelines below, “high-risk” means a BMI of 40 or greater, diabetes, having a medical complication that makes an urgent Cesarean more likely, and/or the possibility of having difficulty managing an airway during anesthesia. In contrast, low-risk would mean the absence of these factors.
Several professional organizations recommend that low-risk birthing people eat or drink as they desire during labor:
-
- The World Health Organization (WHO) (Care in normal birth: a practical Technical Working Group,” 1997)
- The American College of Nurse-Midwives (ACNM) (Providing Oral Nutrition to Women in Labor,” 2016)
- NICE Clinical Guidance for the United Kingdom (Delgado Nunes et 2014)
- The Society of Obstetricians and Gynecologists of Canada (SOGC) (Lee et 2016)
Although Canadian guidelines recommend the option of food and drink, researchers surveyed 118 hospital maternity centers in Canada, and found that most low-risk people are not allowed to eat or drink during active labor (Chackowicz et al. 2016). In early labor, 98% of low-risk laboring people were free to consume fluids and solids. However, in active labor, 60% of people without epidurals and 83% of those with epidurals were restricted to ice chips and clear fluids. The authors concluded with their hope that this study will spark revisions of current hospital policy to be in line with Canadian professional guidelines and best practices and meet “psychological and physiological requirements in labor.”
Other organizations recommend that low-risk people avoid solid food during labor but be free to drink clear liquids, such as water, sports drinks, black coffee, tea, and soda:
-
- The American College of Obstetricians and Gynecologists (ACOG) (“Approaches to Limit Intervention During Labor and Birth,” 2019, Reaffirmed 2021)
- The American Society of Anesthesiologists (ASA) (“Practice Guidelines for Obstetric Anesthesia,” 2016)
In their position statement, the ASA noted that aspiration has become so rare that randomized trials and even large databases have been unable to calculate an incidence:
“There is insufficient evidence to draw conclusions about the relationship between fasting times for clear liquids or solids and the risk of aspiration during delivery.”
In the absence of evidence, the ASA decided to base their guidelines on expert opinion. They conducted an official survey of 357 members, and 77% believed that clear liquids were okay in low-risk laboring people, while 91% said that solid foods should be avoided in all laboring people. So these opinions became the basis of ASA practice guidelines and ACOG’s (Withdrawn) 2009 Committee Opinion No. 441 “Oral Intake During Labor”. Note that it is not evidence-based practice to allow opinions to restrict people’s human rights simply because they think that there is “insufficient evidence.” Insufficient is a vague and subjective term, and as we’ve shown in this Evidence Based Birth® Signature Article, there is a wide range of evidence showing that birthing people can safely eat and drink during labor.
Neither ACOG nor ASA recommends restricting low-risk people to ice chips or sips of water during labor. Providers that continue to enforce NPO policies are not in line with their professional organization’s guidelines. In a recent statement, ACOG’s Committee on Obstetric Practice reaffirms their recommendation to allow people without complications free access to moderate amounts of clear liquids (“Committee Opinion No. 766: Approaches to Limit Intervention During Labor and Birth,” 2019, Reaffirmed 2021). They continue to advise against consuming solid foods while in labor; however, they note that the evidence for this recommendation has been questioned and is under review. To access these guidelines, click here.
AJOG MFM (Maternal-Fetal Medicine), a companion title to the American Journal of Obstetrics and Gynecology, published a recent review of evidence based labor management guidelines (Alhafez and Berghella, 2020). The authors made a strong recommendation that fluid or solid food should not be restricted: “Given that the aspiration risk in uncomplicated women is 1/1,000,000, there is no evidence to support restriction of oral intake.”
In 2009, when ACOG revised its recommendations to allow clear liquids during labor, it was part of a wider trend in the anesthesia community to relax rules on fasting before all surgeries. In a meta- analysis of randomized trials, researchers compared fasting times of two to four hours versus more than four hours and found that the patients who fasted longer were at greater risk of aspiration from larger and more acidic stomach contents (“Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report” 2017). Healthy patients undergoing elective surgeries are now advised to consume clear liquids up until two hours before the procedure, instead of “NPO after midnight.”
Likewise, a recent article inspired by the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely® campaign, entitled “Things We Do for No Reason™: NPO After Midnight,” concluded that NPO after midnight provides little value to surgical patients in general (Black et al. 2021). When patients have scheduled procedures requiring sedation or general anesthesia, requiring NPO after midnight “represents a low-value and arbitrary practice that leaves patients fasting longer than necessary.”
Why is Body Mass Index labeled a “high risk” factor for aspiration?
Having a Body Mass Index (BMI) of greater than 30-40 is sometimes mentioned in the research as making someone at high risk for aspiration.
Here at EBB, we were curious if a higher BMI is related to a true increase in risk of aspiration, or if there is a perception of higher risk due to fatphobia among health care workers (Lee & Pause, 2016).
Fatphobia, also known as being anti-fat, is defined as bias, blame, and stigma against larger people that is rooted in thinner people feeling like they have superior morals. There is also a long history of fatphobia being related to racism and white supremacy—to learn more, visit this NPR podcast episode here.
We could not find any research showing that plus-size people are more likely to experience aspiration during childbirth. So, we looked at a couple of possible sources for this belief, related to intubation and epidural placement.
- We have heard health care workers talk about the difficulty of intubating (providing airway protection) for larger patients. However, research has shown that there is no substantial difference in the difficulty of intubating someone who is larger versus thinner bodied (Shailaja et 2014; Wang et al. 2018).
- Health care workers may also worry about the difficulty of placing an epidural in a plus-size birthing person—leading to a higher risk of needing general anesthesia during a Cesarean. Researchers have found that health care workers have more failed attempts when inserting epidurals in birthing people with a BMI of 40 or higher, due to a difficulty in feeling bony landmarks. However, ultrasound-guided placement of the epidural needle can reduce the length of the procedure and lead to fewer failed attempts (Mossie et al. 2022).
One aspect of fatphobia is when health care providers lack experience or confidence in treating people with diverse size. Thus, the limited evidence we found seems to suggest that the perception of aspiration risk with higher BMI is rooted in fatphobia and not any documented increase in risk.
Bottom Line
In the mid 1900s, when anesthesia methods were crude and unsafe, “Nothing by Mouth” policies came about to prevent the dangerous consequences of aspiration with general anesthesia. Now that the safety of anesthesia has greatly improved, hospital policies and physician guidelines need to be rewritten to be in line with current evidence. We’ve started to see some movement in that direction. Several countries have started encouraging people to eat and drink as desired during labor, and in the U.S., obstetric practice guidelines were updated in 2009 to allow clear liquids.
The research is limited, but fasting as soon as contractions begin may still not guarantee an empty stomach during birth (Carp et al. 1992). Fasting could even be harmful; it could cause stomach juices to become more dangerously acidic if an aspiration were to occur (Harty et al. 2015).
Overall, the Cochrane review of five randomized trials with low-risk participants did not find any evidence for harm or benefit from eating and drinking during labor (Singata et al. 2013). Maybe we would have seen benefits if any of the trials had looked at patient satisfaction—but none of them did.
A larger, more recent review found that the people laboring under less-restrictive eating and drinking policies had shorter labors by about 16 minutes and no other differences in health outcomes (Ciardulli et al. 2017). Only one of the trials in the review considered parental satisfaction and found that more of the eating group participants reported satisfaction with their nourishment during labor compared to those given sips of water only (97% versus 55%) (Goodall & Wallymahmed, 2006).
The issue of eating and drinking during labor should be reframed as one of bodily autonomy and human rights. All laboring people, whether they have an epidural or not, or have diabetes or not, have the right to choose whether they would like to eat and drink during labor.
Here at Evidence Based Birth®, we urge the American Society of Anesthesiologists and the American College of Obstetricians and Gynecologists to revisit their current guidelines and adjust them according to the evidence and ethics. Any revisions in guidelines should also consider parental satisfaction. There is abundant research showing that people often complain about their distress in being forced to fast during labor (Manizheh & Leila, 2009).
In high-risk situations, the informed consent discussion might look a bit different. People should know there is no evidence from randomized trials that could be applied to a higher-risk situation (and having a high BMI should not mean that you are automatically “high risk”). However, regardless of risk status, people should not have food and drink withheld against their will.


References:
- The American Association of Clinical Endocrinology (AACE). Glycemic Control During Labor and Delivery. Accessed online April 29, 2022. Available at: https://pro.aace.com/ disease-state-resources/diabetes/depth-information/ glycemic-control-during-labor-and-delivery
- ACOG (2019, Reaffirmed 2021). Committee Opinion No. 766: Approaches to Limit Intervention During Labor and Birth. Obstet Gynecol. 2019 Feb;133(2):e164-e173.
- ACOG (2018). ACOG Practice Bulletin No. 201: Pregestational Diabetes Mellitus. Obstet Gynecol. 2018 Dec;132(6):e228-e248.
- Alhafez, L.and Berghella, V. (2020). Evidence-based Labor Management: First stage of labor (Part 3), American Journal of Obstetrics & Gynecology MFM.
- Bataille, A., Rousset, J., Marret, E., & Bonnet, F. (2014). Ultrasonographic evaluation of gastric content during labour under epidural analgesia: a prospective cohort study. Br J Anaesth, 112(4), 703-707.
- Begley, C., Sedlicka, N. & Daly, D. (2018). Respectful and disrespectful care in the Czech Republic: an online survey. Reprod Health 15, 198.
- Black, M. K., Lupa, M. C., Lemley, L. W., et al. (2021). Things We Do for No Reason™: NPO After Midnight. J Hosp Med. 2021 Jun;16(6):368-370.
- Bouvet, L., Schulz, T., Piana, F., et al. (2022). Pregnancy and Labor Epidural Effects on Gastric Emptying: A Prospective Comparative Study. Anesthesiology. 2022 Apr 1;136(4):542-550.
- Cantwell, R., Clutton-Brock, T., Cooper, G., Dawson, A., Drife, J., Garrod, D., . . . Springett, A. (2011). Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG, 118 Suppl 1, 1-203.
- Care in normal birth: a practical guide. Technical Working Group, World Health Organization. (1997). Birth, 24(2), 121-123.
- Carp, H., Jayaram, A., & Stoll, M. (1992). Ultrasound examination of the stomach contents of parturients. Anesth Analg, 74(5), 683-687.
- Chackowicz, A., Spence, A. R., & Abenhaim, H. A. (2016). Restrictions on Oral and Parenteral Intake for Low-risk Labouring Women in Hospitals Across Canada: A Cross- Sectional Study. J Obstet Gynaecol Can, 38(11), 1009-1014.
- Ciardulli, A., Saccone, G., Anastasio, H., & Berghella, V. (2017). Less-Restrictive Food Intake During Labor in Low- Risk Singleton Pregnancies: A Systematic Review and Meta-analysis. Obstet Gynecol.
- Cook, T. M., Woodall, N., Frerk, C., & Project, F. N. A. (2011). Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth, 106(5), 617-631.
- D’Angelo, R., Smiley, R. M., Riley, E. T., & Segal, S. (2014). Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology, 120(6), 1505-1512.
- Declercq, E. R., Sakala, C., Corry, M. P., Applebaum, S., & Herrlich, A. (2014). Major Survey Findings of Listening to Mothers(SM) III: Pregnancy and Birth: Report of the Third National U.S. Survey of Women’s Childbearing Experiences. J Perinat Educ, 23(1), 9-16.
- Delgado Nunes, V., Gholitabar, M., Sims, J. M., Bewley, S., & Group, G. D. (2014). Intrapartum care of healthy women and their babies: summary of updated NICE guidance. BMJ, 349, g6886.
- Goodall, U., & Wallymahmed, A. H. (2006). Unpublished.
- Harty, C., Sproul, E., Bautista, M. J., Major, A. E., & Farrell, (2015). A Review of Fasting and the Risk of Aspiration in Labour. Memorial University Faculty of Medicine, St. John’s Newfoundland and Labrador, Canada. American Society of Anesthesiologists ABSTRACT.
- Hawkins, J. L., Chang, J., Palmer, S. K., Gibbs, C. P., & Callaghan, W. M. (2011). Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol, 117(1), 69-74.
- Hawkins, J. L., Koonin, L. M., Palmer, S. K., & Gibbs, C. P. (1997). Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology, 86(2), 277-284.
- Huang, C. Y., Luo, B. R. and Hu, J. (2020). Investigation on the status of oral intake management measures during labor in China. Medicine (Baltimore). 2020 Jun 5;99(23):e20626.
- Iravani, M., Zarean, E., Janghorbani, M., & Bahrami, M. (2015). Women’s needs and expectations during normal labor and delivery. J Educ Health Promot, 4, 6.
- Knight, M., Bogod, D., Lucas, D.N., et al. (2016). Pulmonary aspiration during pregnancy or immediately postpartum in the UK: a two-year national descriptive study. International Journal of Obstetric Anesthesia 26(Supplement 1): S6-S54.
- Knight, M., Nair, M., Tuffnell, D., et al. (2017) on behalf of MBRRACE-UK. Saving Lives, Improving Mothers’ Care – Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2013–15. Oxford: National Perinatal Epidemiology Unit, University of Oxford 2017.
- Kordi, M., Salek Nasiri, N., Safari, M., Esmaeili, H., & Shadjuo, K. (2010). The effect of oral honey-date syrup intake during labor on labor progress of nulliparous women. Iranian J Obstet Gynecol Infertility, 13, 23-30.
- Kubli, M., Scrutton, M. J., Seed, P. T., & O’Sullivan, G. (2002). An evaluation of isotonic “sport drinks” during labor. Anesth Analg, 94(2), 404-408.
- Laifer, S. A., Siddiqui, T. P., Collins, J. E., Stiller, R. J., Moffat, S. L., Loh, E. V., . . . Buonafede, D. S. (2000). A Prospective Randomized Controlled Trial of Oral Intake of Liquids During the First Stage of Labor [abstract]. Paper presented at the Anesthesiology (Poster 12). Unpublished.
- Lee, L., Dy, J., & Azzam, H. (2016). Practice Guidelines for the Society of Obstetricians and Gynaecologists of Canada: Management of Spontaneous Labour at Term in Healthy Women J Obstet Gynaecol Can, 38(9), 843- 865.
- Liang, G., Bhiladvala, C. and Preston, R. (2021). Nutritional preferences of women during labour: a survey study. Int J Obstet Anesth. 2021 Nov;48:103209.
- Lucas, D. N. and Bamber, J. H. (2018). UK Confidential Enquiry into Maternal Deaths – still learning to save mothers’ lives. Anaesthesia. 2018 Apr;73(4):416-420.
- Manizheh, P., & Leila, P. (2009). Perceived environmental stressors and pain perception during labor among primiparous and multiparous women. J Reprod Infertil, 10(3), 217-223.
- McDermott, L., Pelecanos, A., Krepska, A., et al. (2022). Single-centre survey of women reflecting on recent experiences and preferences of oral intake during labour. Aust N Z J Obstet Gynaecol. 2022 Mar 27. Epub ahead of print.
- Mendelson, C. L. (1946). The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol, 52, 191-205.
- Mhyre, J. M., Riesner, M. N., Polley, L. S., & Naughton,
- N. (2007). A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology, 106(6), 1096-1104.
- Mhyre, J. M., Tsen, L. C., Einav, S., Kuklina, E. V., Leffert, R., & Bateman, B. T. (2014). Cardiac arrest during hospitalization for delivery in the United States, 1998- 2011. Anesthesiology, 120(4), 810-818.
- Mossie, A. M., Ali, S. A., Tesema, H. G. (2022). Anesthetic implications of morbid obesity during pregnancy: a literature based review. Int J Surgery Open 40: 100444.
- O’Sullivan, G., Liu, B., Hart, D., Seed, P., & Shennan, A. (2009). Effect of food intake during labour on obstetric outcome: randomised controlled trial. BMJ, 338, b784.
- Palmer, C. M. and Jiang, Y. (2022). Limiting Oral Intake during Labor: Do We Have It Right?. Anesthesiology 2022; 136:528–530.
- Practice Guidelines for Obstetric Anesthesia: An Updated Report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. (2016). Anesthesiology, 124(2), 270-300.
- Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. (2017). Anesthesiology, 126(3), 376-393.
- Providing Oral Nutrition to Women in Labor: American College of Nurse-Midwives. (2016). J Midwifery Womens Health, 61(4), 528-534.
- Rahmani, R., Khakbazan, Z., Yavari, P., Granmayeh, M., & Yavari, L. (2012). Effect of oral carbohydrate intake on labor progress: randomized controlled trial. Iran J Public Health, 41(11), 59-66.
- Robinson, D. H., & Toledo, A. H. (2012). Historical development of modern anesthesia. J Invest Surg, 25(3), 141-149.
- Rodriguez, N. R., Di Marco, N. M., Langley, S., Association, D., Canada, D. o., & Medicine, A. C. o. S. (2009). American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc, 41(3), 709-731.
- Rooks, J. P., Weatherby, N. L., Ernst, E. K., Stapleton, S., Rosen, D., & Rosenfield, A. (1989). Outcomes of care in birth centers. The National Birth Center Study. N Engl J Med, 321(26), 1804-1811.
- Scheepers, H. C., de Jong, P. A., Essed, G. G., & Kanhai, H. (2004). Carbohydrate solution intake during labour just before the start of the second stage: a double-blind study on metabolic effects and clinical outcome. BJOG, 111(12), 1382-1387.
- Scheepers, H. C., Thans, M. C., de Jong, P. A., Essed, G. , Le Cessie, S., & Kanhai, H. H. (2002). A double-blind, randomised, placebo controlled study on the influence of carbohydrate solution intake during labour. BJOG, 109(2), 178-181.
- Scrutton, M. J., Metcalfe, G. A., Lowy, C., Seed, P. T., & O’Sullivan, G. (1999). Eating in labour. A randomised controlled trial assessing the risks and benefits. Anaesthesia, 54(4), 329-334.
- Serbin, J.W., & Donnelly, S. (2016). The impact of racism and midwifery’s lack of racial diversity: a literature review. J Midwifery Womens Health 61: 694-706.
- Shailaja, S., Nichelle, S. M., Shetty, A. K., et al. (2014). Comparing ease of intubation in obese and lean patients using intubation difficulty scale. Anesth Essays Res 8(2): 168–174.
- Singata, M., Tranmer, J., & Gyte, G. M. L. (2013). Restricting oral fluid and food intake during labour. Cochrane Database of Systematic Reviews(8).
- Sperling, J. D., Dahlke, J. D., & Sibai, B. M. (2016). Restriction of oral intake during labor: whither are we bound? Am J Obstet Gynecol, 214(5), 592-596.
- Toohill, J., Soong, B., & Flenady, V. (2008). Interventions for ketosis during labour. Cochrane Database Syst Rev(3), CD004230.
- Tranmer, J. E., Hodnett, E. D., Hannah, M. E., & Stevens, B. J. (2005). The effect of unrestricted oral carbohydrate intake on labor progress. J Obstet Gynecol Neonatal Nurs, 34(3), 319-328. doi:10.1177/0884217505276155.
- S. Department of Health and Human Services, Health Resources and Services Administration (HRSA), National Center for Health Workforce Analysis. (2017). Sex, race, and ethnic diversity of U.S., health occupations (2011- 2015). Rockville, MD.
- Wang, T., Sun, S., & Huang, S. (2018). The association of body mass index with difficult tracheal intubation management by direct laryngoscopy: a meta-analysis. BMC Anesthesiology 18: 79.
Acknowledgment
We would like to extend our gratitude to our expert clinician reviewers for their feedback on the 2017 version of this article: Claudia Breglia, LM, CPM, attends births at home and The Natural Birth and Women’s Center in Canoga Park, CA; Rebecca Bull, MD, practices Family Medicine with Obstetrics in Madison WI since 1989; Saraswathi Vedam, RM, FACNM, MSN, Sci D(hc), Associate Professor at the University of British Columbia; Shannon J. Voogt, MD, Board-Certified in Family Medicine; and Kathy Watkins, RN, BSN, MSN, CNM, practices full scope midwifery providing locum relief to hospitals, and birth centers.
Birth Professionals:
Join others who also want to help bring evidence-based care to their local community.
Also, gain complimentary access to a printable library of our Signature Articles, 20+ hours of CE courses, a private community, and more.
Buy EBB Inspirational T-shirts, Due Date Buttons & Birth Affirmation Cards




Stay empowered, read more :
EBB 308 – The Intersection of Environmental Justice and Midwifery Care with Dr. Tanya Khemet Taiwo
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | YouTube Curious which toxins should be most avoided for people of reproductive age and their children? In this episode, Dr. Tanya Khemet Taiwo, LM, CPM, MPH, PhD unravels the...
EBB 307 – Unexplained Infertility, Endometriosis, and a Birth Center Birth Story with Ellora La Shier, EBB Childbirth Class Graduate
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | GoogleIn this episode, Ellora La Shier, graduate of the EBB Childbirth Class, shares about her struggle with six years of unexplained infertility and how it impacted her...
EBB 306 – Culturally Sensitive Doula Training with Divya Deswal and Neha Misra of the Doula Collective in India
Don't miss an episode! Subscribe to our podcast and leave a review! iTunes | Spotify | Google As we wrap up the celebration of World Doula Week, explore the transformative journey of pregnancy and childbirth in the Indian context with Divya Deswal and Neha Misra,...



